Adhesion-Based Self-Organization in Tissue Patterning
- PMID: 35562853
- PMCID: PMC9547846
- DOI: 10.1146/annurev-cellbio-120420-100215
Adhesion-Based Self-Organization in Tissue Patterning
Abstract
Since the proposal of the differential adhesion hypothesis, scientists have been fascinated by how cell adhesion mediates cellular self-organization to form spatial patterns during development. The search for molecular tool kits with homophilic binding specificity resulted in a diverse repertoire of adhesion molecules. Recent understanding of the dominant role of cortical tension over adhesion binding redirects the focus of differential adhesion studies to the signaling function of adhesion proteins to regulate actomyosin contractility. The broader framework of differential interfacial tension encompasses both adhesion and nonadhesion molecules, sharing the common function of modulating interfacial tension during cell sorting to generate diverse tissue patterns. Robust adhesion-based patterning requires close coordination between morphogen signaling, cell fate decisions, and changes in adhesion. Current advances in bridging theoretical and experimental approaches present exciting opportunities to understand molecular, cellular, and tissue dynamics during adhesion-based tissue patterning across multiple time and length scales.
Keywords: cell adhesion; cell sorting; developmental biology; interfacial tension; tissue patterning.
Figures
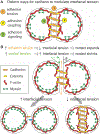
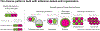

References
-
- Amack JD, and Manning ML (2012). Knowing the Boundaries: Extending the Differential Adhesion Hypothesis in Embryonic Cell Sorting. Science 338, 212–215. - PubMed
-
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, and Reynolds AB (2000). Inhibition of RhoA by p120 catenin. Nat Cell Biol 2, 637–644. - PubMed
-
- Barone V, Lang M, Krens SFG, Pradhan SJ, Shamipour S, Sako K, Sikora M, Guet CC, and Heisenberg C-P (2017). An Effective Feedback Loop between Cell-Cell Contact Duration and Morphogen Signaling Determines Cell Fate. Developmental Cell 43, 198–211.e12. - PubMed
-
- Bekirov IH, Needleman LA, Zhang W, and Benson DL (2002). Identification and localization of multiple classic cadherins in developing rat limbic system. Neuroscience 115, 213–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

