Examination of the Impact of CYP3A4/5 on Drug-Drug Interaction between Schizandrol A/Schizandrol B and Tacrolimus (FK-506): A Physiologically Based Pharmacokinetic Modeling Approach
- PMID: 35562875
- PMCID: PMC9103789
- DOI: 10.3390/ijms23094485
Examination of the Impact of CYP3A4/5 on Drug-Drug Interaction between Schizandrol A/Schizandrol B and Tacrolimus (FK-506): A Physiologically Based Pharmacokinetic Modeling Approach
Abstract
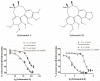
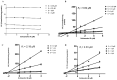
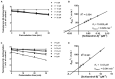
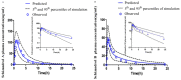
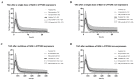
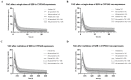
Schizandrol A (SZA) and schizandrol B (SZB) are two active ingredients of Wuzhi capsule (WZC), a Chinese proprietary medicine commonly prescribed to alleviate tacrolimus (FK-506)-induced hepatoxicity in China. Due to their inhibitory effects on cytochrome P450 (CYP) 3A enzymes, SZA/SZB may display drug-drug interaction (DDI) with tacrolimus. To identify the extent of this DDI, the enzymes' inhibitory profiles, including a 50% inhibitory concentration (IC50) shift, reversible inhibition (RI) and time-dependent inhibition (TDI) were examined with pooled human-liver microsomes (HLMs) and CYP3A5-genotyped HLMs. Subsequently, the acquired parameters were integrated into a physiologically based pharmacokinetic (PBPK) model to quantify the interactions between the SZA/SZB and the tacrolimus. The metabolic studies indicated that the SZB displayed both RI and TDI on CYP3A4 and CYP3A5, while the SZA only exhibited TDI on CYP3A4 to a limited extent. Moreover, our PBPK model predicted that multiple doses of SZB would increase tacrolimus exposure by 26% and 57% in CYP3A5 expressers and non-expressers, respectively. Clearly, PBPK modeling has emerged as a powerful approach to examine herb-involved DDI, and special attention should be paid to the combined use of WZC and tacrolimus in clinical practice.
Keywords: CYP3A5 polymorphism; Wuzhi capsule (WZC); drug–drug interaction (DDI); physiologically based pharmacokinetic (PBPK); schizandrol A (SZA); schizandrol B (SZB); tacrolimus (FK-506).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gonschior A., Christians U., Braun F., Winkler M., Linck A., Baumann J., Sewing K. Measurement of blood concentrations of FK506 (tacrolimus) and its metabolites in seven liver graft patients after the first dose by h.p.l.c.-MS and microparticle enzyme immunoassay (MEIA) Br. J. Clin. Pharmacol. 1994;38:567–571. doi: 10.1111/j.1365-2125.1994.tb04398.x. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

