Alleviation of Endoplasmic Reticulum Stress Enhances Human Corneal Epithelial Cell Viability under Hyperosmotic Conditions
- PMID: 35562919
- PMCID: PMC9104051
- DOI: 10.3390/ijms23094528
Alleviation of Endoplasmic Reticulum Stress Enhances Human Corneal Epithelial Cell Viability under Hyperosmotic Conditions
Abstract
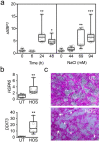
Tear hyperosmolarity plays an essential role in the initiation and progression of dry-eye disease. Under a hyperosmotic environment, corneal epithelial cells experience perturbations in endoplasmic reticulum function that can lead to proinflammatory signaling and apoptosis. In this study, we investigated the effect of tauroursodeoxycholic acid (TUDCA), a chemical chaperone known to protect against endoplasmic reticulum stress, on corneal epithelial cells exposed to hyperosmotic conditions. We found that the expression of the genes involved in the activation of the unfolded protein response and the pro-apoptotic transcription factor DDIT3 were markedly upregulated in patients with Sjögren's dry-eye disease and in a human model of corneal epithelial differentiation following treatment with hyperosmotic saline. Experiments in vitro demonstrated that TUDCA prevented hyperosmotically induced cell death by reducing nuclear DNA fragmentation and caspase-3 activation. TUDCA supplementation also led to the transcriptional repression of CXCL8 and IL5, two inflammatory mediators associated with dry-eye pathogenesis. These studies highlight the role of hyperosmotic conditions in promoting endoplasmic reticulum stress in the cornea and identify TUDCA as a potential therapeutic agent for the treatment of dry-eye disease.
Keywords: corneal epithelium; dry eye; endoplasmic reticulum stress; hyperosmotic stress; tauroursodeoxycholic acid; unfolded protein response.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Coursey T.G., Tukler Henriksson J., Barbosa F.L., de Paiva C.S., Pflugfelder S.C. Interferon-γ-Induced Unfolded Protein Response in Conjunctival Goblet Cells as a Cause of Mucin Deficiency in Sjögren Syndrome. Am. J. Pathol. 2016;186:1547–1558. doi: 10.1016/j.ajpath.2016.02.004. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

