Demystifying the Neuroprotective Role of Neuropeptides in Parkinson's Disease: A Newfangled and Eloquent Therapeutic Perspective
- PMID: 35562956
- PMCID: PMC9099669
- DOI: 10.3390/ijms23094565
Demystifying the Neuroprotective Role of Neuropeptides in Parkinson's Disease: A Newfangled and Eloquent Therapeutic Perspective
Abstract
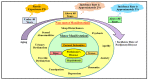
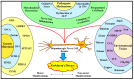
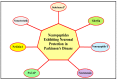
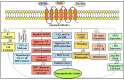
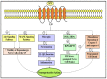
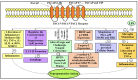
Parkinson's disease (PD) refers to one of the eminently grievous, preponderant, tortuous nerve-cell-devastating ailments that markedly impacts the dopaminergic (DArgic) nerve cells of the midbrain region, namely the substantia nigra pars compacta (SN-PC). Even though the exact etiopathology of the ailment is yet indefinite, the existing corroborations have suggested that aging, genetic predisposition, and environmental toxins tremendously influence the PD advancement. Additionally, pathophysiological mechanisms entailed in PD advancement encompass the clumping of α-synuclein inside the lewy bodies (LBs) and lewy neurites, oxidative stress, apoptosis, neuronal-inflammation, and abnormalities in the operation of mitochondria, autophagy lysosomal pathway (ALP), and ubiquitin-proteasome system (UPS). The ongoing therapeutic approaches can merely mitigate the PD-associated manifestations, but until now, no therapeutic candidate has been depicted to fully arrest the disease advancement. Neuropeptides (NPs) are little, protein-comprehending additional messenger substances that are typically produced and liberated by nerve cells within the entire nervous system. Numerous NPs, for instance, substance P (SP), ghrelin, neuropeptide Y (NPY), neurotensin, pituitary adenylate cyclase-activating polypeptide (PACAP), nesfatin-1, and somatostatin, have been displayed to exhibit consequential neuroprotection in both in vivo and in vitro PD models via suppressing apoptosis, cytotoxicity, oxidative stress, inflammation, autophagy, neuronal toxicity, microglia stimulation, attenuating disease-associated manifestations, and stimulating chondriosomal bioenergetics. The current scrutiny is an effort to illuminate the neuroprotective action of NPs in various PD-experiencing models. The authors carried out a methodical inspection of the published work procured through reputable online portals like PubMed, MEDLINE, EMBASE, and Frontier, by employing specific keywords in the subject of our article. Additionally, the manuscript concentrates on representing the pathways concerned in bringing neuroprotective action of NPs in PD. In sum, NPs exert substantial neuroprotection through regulating paramount pathways indulged in PD advancement, and consequently, might be a newfangled and eloquent perspective in PD therapy.
Keywords: Parkinson’s disease; ghrelin; neuropeptide Y; neuropeptides; neuroprotective action; neurotensin; pituitary adenylate cyclase-activating polypeptide; substance P.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Dawson T.M. Parkinson’s Disease: Clinical Manifestations and Treatment. Int. Rev. Psychiatry. 2000;12:263–269. doi: 10.1080/09540260020002488. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

