A Novel β-Hairpin Peptide Z-d14CFR Enhances Multidrug-Resistant Bacterial Clearance in a Murine Model of Mastitis
- PMID: 35563007
- PMCID: PMC9105976
- DOI: 10.3390/ijms23094617
A Novel β-Hairpin Peptide Z-d14CFR Enhances Multidrug-Resistant Bacterial Clearance in a Murine Model of Mastitis
Abstract
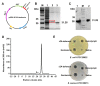
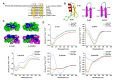
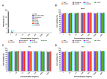
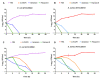
The widespread prevalence of antimicrobial resistance has spawned the development of novel antimicrobial agents. Antimicrobial peptides (AMPs) have gained comprehensive attention as one of the major alternatives to antibiotics. However, low antibacterial activity and high-cost production have limited the applications of natural AMPs. In this study, we successfully expressed recombinant Zophobas atratus (Z. atratus) defensin for the first time. In order to increase the antimicrobial activity of peptide, we designed 5 analogues derived from Z. atratus defensin, Z-d13, Z-d14C, Z-d14CF, Z-d14CR and Z-d14CFR. Our results showed that Z-d14CFR (RGCRCNSKSFCVCR-NH2) exhibited a broad-spectrum antimicrobial activity to both Gram-positive bacteria and Gram-negative bacteria, including multidrug-resistant bacteria. It possessed less than 5% hemolysis and 10% cytotoxicity, even at a high concentration of 1 mg/mL. Antimicrobial mechanism studies indicated that Z-d14CFR performed antimicrobial effect via inhibiting biofilm formation, disrupting bacterial membrane integrity and inducing cellular contents release. Furthermore, Z-d14CFR showed a great therapeutic effect on the treatment of multidrug-resistant Escherichia coli (E. coli) infection by enhancing bacterial clearance, decreasing neutrophils infiltration and the expression of tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) in a murine model of mastitis. Our findings suggest that Z-d14CFR could be a promising candidate against multidrug-resistant bacteria.
Keywords: Zophobas atratus; antimicrobial peptide; defensin; mastitis; multidrug-resistant bacteria; β-hairpin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
A novel glycine-rich peptide from Zophobas atratus, coleoptericin B, targets bacterial membrane and protects against Klebsiella pneumoniae-induced mastitis in mice.J Antimicrob Chemother. 2024 Feb 1;79(2):417-428. doi: 10.1093/jac/dkad397. J Antimicrob Chemother. 2024. PMID: 38267384
-
Self-assembly antimicrobial peptide for treatment of multidrug-resistant bacterial infection.J Nanobiotechnology. 2024 Oct 30;22(1):668. doi: 10.1186/s12951-024-02896-5. J Nanobiotechnology. 2024. PMID: 39478570 Free PMC article.
-
Membrane mechanism of temporin-1CEc, an antimicrobial peptide isolated from the skin secretions of Rana chensinensis, and its systemic analogs.Bioorg Chem. 2022 Feb;119:105544. doi: 10.1016/j.bioorg.2021.105544. Epub 2021 Dec 8. Bioorg Chem. 2022. PMID: 34953322
-
Antimicrobial peptides as potential anti-biofilm agents against multidrug-resistant bacteria.J Microbiol Immunol Infect. 2017 Aug;50(4):405-410. doi: 10.1016/j.jmii.2016.12.005. Epub 2017 Jun 26. J Microbiol Immunol Infect. 2017. PMID: 28690026 Review.
-
Antimicrobial Peptides: A Promising Alternative to Conventional Antimicrobials for Combating Polymicrobial Biofilms.Adv Sci (Weinh). 2025 Jan;12(1):e2410893. doi: 10.1002/advs.202410893. Epub 2024 Nov 12. Adv Sci (Weinh). 2025. PMID: 39530703 Free PMC article. Review.
Cited by
-
A New Approach for Phage Cocktail Design in the Example of Anti-Mastitis Solution.Pathogens. 2024 Sep 27;13(10):839. doi: 10.3390/pathogens13100839. Pathogens. 2024. PMID: 39452711 Free PMC article.
-
Revealing the Diversity of Sequences, Structures, and Targets of Peptides from South China Sea Macrodactyla doreensis Based on Transcriptomics.Mar Drugs. 2024 Oct 12;22(10):470. doi: 10.3390/md22100470. Mar Drugs. 2024. PMID: 39452877 Free PMC article.
-
The Contribution of Antimicrobial Peptides to Immune Cell Function: A Review of Recent Advances.Pharmaceutics. 2023 Sep 4;15(9):2278. doi: 10.3390/pharmaceutics15092278. Pharmaceutics. 2023. PMID: 37765247 Free PMC article. Review.
-
Synergistic Efficacy of Doxycycline and Florfenicol Against Aeromonas hydrophilia and Morganella morganii Infections in Pelodiscus sinensis with Skin Ulcer Disease.Vet Sci. 2025 Jun 23;12(7):611. doi: 10.3390/vetsci12070611. Vet Sci. 2025. PMID: 40711271 Free PMC article.
-
Designing, Synthesis and In Vitro Antimicrobial Activity of Peptide Against Biofilm Forming Methicillin Resistant Staphylococcus aureus.Curr Microbiol. 2025 Feb 27;82(4):159. doi: 10.1007/s00284-025-04132-1. Curr Microbiol. 2025. PMID: 40014067
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

