Molecular and Signaling Mechanisms for Docosahexaenoic Acid-Derived Neurodevelopment and Neuroprotection
- PMID: 35563025
- PMCID: PMC9100376
- DOI: 10.3390/ijms23094635
Molecular and Signaling Mechanisms for Docosahexaenoic Acid-Derived Neurodevelopment and Neuroprotection
Abstract
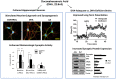
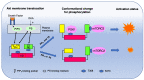
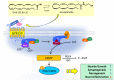
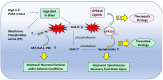
The neurodevelopmental and neuroprotective actions of docosahexaenoic acid (DHA) are mediated by mechanisms involving membrane- and metabolite-related signal transduction. A key characteristic in the membrane-mediated action of DHA results from the stimulated synthesis of neuronal phosphatidylserine (PS). The resulting DHA-PS-rich membrane domains facilitate the translocation and activation of kinases such as Raf-1, protein kinase C (PKC), and Akt. The activation of these signaling pathways promotes neuronal development and survival. DHA is also metabolized in neural tissues to bioactive mediators. Neuroprotectin D1, a docosatriene synthesized by the lipoxygenase activity, has an anti-inflammatory property, and elovanoids formed from DHA elongation products exhibit antioxidant effects in the retina. Synaptamide, an endocannabinoid-like lipid mediator synthesized from DHA in the brain, promotes neurogenesis and synaptogenesis and exerts anti-inflammatory effects. It binds to the GAIN domain of the GPR110 (ADGRF1) receptor, triggers the cAMP/protein kinase A (PKA) signaling pathway, and activates the cAMP-response element binding protein (CREB). The DHA status in the brain influences not only the PS-dependent signal transduction but also the metabolite formation and expression of pre- and post-synaptic proteins that are downstream of the CREB and affect neurotransmission. The combined actions of these processes contribute to the neurodevelopmental and neuroprotective effects of DHA.
Keywords: ADGRF1; Akt; GPR110; N-docosahexaenoylethanolamine; N-docosahexaenoylphosphatidylethanolamine; PKA; cAMP; docosahexaenoic acid; phosphatidylserine; synaptamide; synaptic membrane proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

