Effects of Gamma-Tocotrienol on Intestinal Injury in a GI-Specific Acute Radiation Syndrome Model in Nonhuman Primate
- PMID: 35563033
- PMCID: PMC9100017
- DOI: 10.3390/ijms23094643
Effects of Gamma-Tocotrienol on Intestinal Injury in a GI-Specific Acute Radiation Syndrome Model in Nonhuman Primate
Abstract
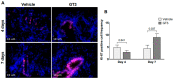
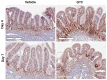
The gastrointestinal (GI) system is highly susceptible to irradiation. Currently, there is no Food and Drug Administration (FDA)-approved medical countermeasures for GI radiation injury. The vitamin E analog gamma-tocotrienol (GT3) is a promising radioprotector in mice and nonhuman primates (NHP). We evaluated GT3-mediated GI recovery in total-body irradiated (TBI) NHPs. Sixteen rhesus macaques were divided into two groups; eight received vehicle and eight GT3 24 h prior to 12 Gy TBI. Proximal jejunum was assessed for structural injuries and crypt survival on day 4 and 7. Apoptotic cell death and crypt cell proliferation were assessed with TUNEL and Ki-67 immunostaining. Irradiation induced significant shortening of the villi and reduced mucosal surface area. GT3 induced an increase in crypt depth at day 7, suggesting that more stem cells survived and proliferated after irradiation. GT3 did not influence crypt survival after irradiation. GT3 treatment caused a significant decline in TUNEL-positive cells at both day 4 (p < 0.03) and 7 (p < 0.0003). Importantly, GT3 induced a significant increase in Ki-67-positive cells at day 7 (p < 0.05). These data suggest that GT3 has radioprotective function in intestinal epithelial and crypt cells. GT3 should be further explored as a prophylactic medical countermeasure for radiation-induced GI injury.
Keywords: gamma-tocotrienol; intestine; nonhuman primates; radiation; radiation countermeasure.
Conflict of interest statement
Authors declare no conflict of interest and they alone are responsible for the content and writing of this manuscript.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

