Acute Hypoxia Alters Extracellular Vesicle Signatures and the Brain Citrullinome of Naked Mole-Rats (Heterocephalus glaber)
- PMID: 35563075
- PMCID: PMC9100269
- DOI: 10.3390/ijms23094683
Acute Hypoxia Alters Extracellular Vesicle Signatures and the Brain Citrullinome of Naked Mole-Rats (Heterocephalus glaber)
Abstract
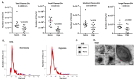
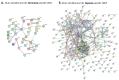
Peptidylarginine deiminases (PADs) and extracellular vesicles (EVs) may be indicative biomarkers of physiological and pathological status and adaptive responses, including to diseases and disorders of the central nervous system (CNS) and related to hypoxia. While these markers have been studied in hypoxia-intolerant mammals, in vivo investigations in hypoxia-tolerant species are lacking. Naked mole-rats (NMR) are among the most hypoxia-tolerant mammals and are thus a good model organism for understanding natural and beneficial adaptations to hypoxia. Thus, we aimed to reveal CNS related roles for PADs in hypoxia tolerance and identify whether circulating EV signatures may reveal a fingerprint for adaptive whole-body hypoxia responses in this species. We found that following in vivo acute hypoxia, NMR: (1) plasma-EVs were remodelled, (2) whole proteome EV cargo contained more protein hits (including citrullinated proteins) and a higher number of associated KEGG pathways relating to the total proteome of plasma-EVs Also, (3) brains had a trend for elevation in PAD1, PAD3 and PAD6 protein expression, while PAD2 and PAD4 were reduced, while (4) the brain citrullinome had a considerable increase in deiminated protein hits with hypoxia (1222 vs. 852 hits in normoxia). Our findings indicate that circulating EV signatures are modified and proteomic content is reduced in hypoxic conditions in naked mole-rats, including the circulating EV citrullinome, while the brain citrullinome is elevated and modulated in response to hypoxia. This was further reflected in elevation of some PADs in the brain tissue following acute hypoxia treatment. These findings indicate a possible selective role for PAD-isozymes in hypoxia response and tolerance.
Keywords: Heterocephalus glaber; KEGG; central nervous system; deimination/citrullination; extracellular vesicles (EVs); hypoxia; in vivo; peptidylarginine deiminase (PAD); plasma.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Buffenstein R., Amoroso V., Andziak B., Avdieiev S., Azpurua J., Barker A.J., Bennett N.C., Brieno-Enriquez M.A., Bronner G.N., Coen C., et al. The naked truth: A comprehensive clarification and classification of current ‘myths’ in naked mole-rat biology. Biol. Rev. Camb. Philos. Soc. 2022;97:115–140. doi: 10.1111/brv.12791. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

