Evaluation of a Gel Containing a Propionibacterium Extract in an In Vivo Model of Wound Healing
- PMID: 35563099
- PMCID: PMC9101165
- DOI: 10.3390/ijms23094708
Evaluation of a Gel Containing a Propionibacterium Extract in an In Vivo Model of Wound Healing
Abstract
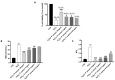
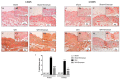
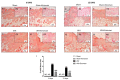
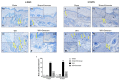
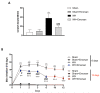
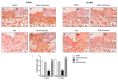
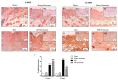
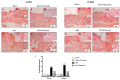
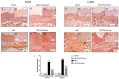
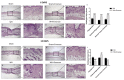
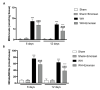
Inappropriate wound healing (WH) management can cause significant comorbidities, especially in patients affected by chronic and metabolic diseases, such as diabetes. WH involves several different, partially overlapping processes, including hemostasis, inflammation, cell proliferation, and remodeling. Oxidative stress in WH contributes to WH impairment because of the overexpression of radical oxygen species (ROS) and nitrogen species (RNS). This study aimed to evaluate the in vitro antioxidative action of a gel containing a Propionibacterium extract (Emorsan® Gel) and assess its skin re-epithelialization properties in a mouse model of WH. The scavenging effects of the bacterial extract were assessed in vitro through the ABTS and DPPH assays and in L-929 murine fibroblasts. The effects of the Emorsan® Gel were studied in vivo in a murine model of WH. After WH induction, mice were treated daily with vehicle or Emorsan® Gel for 6 or 12 days. According to the in vitro tests, the Propionibacterium extract exerted an inhibitory effect on ROS and RNS, consequently leading to the reduction in malondialdehyde (MDA) and nitrite levels. Before proceeding with the in vivo study, the Emorsan® Gel was verified to be unabsorbed. Therefore, the observed effects could be ascribed to a local action. The results obtained in vivo showed that through local reduction of oxidative stress and inflammation (IL-1β, TNF-α), the Emorsan® Gel significantly reduced the infiltration of mast cells into the injured wound, leading to the amelioration of symptoms such as itch and skin irritation. Therefore, the Emorsan® Gel improved the speed and percentage of wound area closure by improving the tissue remodeling process, prompting vascular-endothelial growth factor (VEGF) and transforming growth factor (TGF)- β production and reducing the expression of adhesion molecules. Emorsan® Gel, by its ability to inhibit free radicals, could reduce local inflammation and oxidative stress, thus enhancing the speed of wound healing.
Keywords: Emorsan® Gel; Propionibacterium extract; anti-inflammatory effect; antioxidant effect; bacterial lysate; skin care; topical treatment; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

