Synthesis of C20-38 Fatty Acids in Plant Tissues
- PMID: 35563119
- PMCID: PMC9101283
- DOI: 10.3390/ijms23094731
Synthesis of C20-38 Fatty Acids in Plant Tissues
Abstract
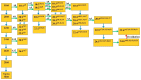
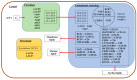
Very-long-chain fatty acids (VLCFA) are involved in a number of important plant physiological functions. Disorders in the expression of genes involved in the synthesis of VLCFA lead to a number of phenotypic consequences, ranging from growth retardation to the death of embryos. The elongation of VLCFA in the endoplasmic reticulum (ER) is carried out by multiple elongase complexes with different substrate specificities and adapted to the synthesis of a number of products required for a number of metabolic pathways. The information about the enzymes involved in the synthesis of VLCFA with more than 26 atoms of Carbon is rather poor. Recently, genes encoding enzymes involved in the synthesis of both regular-length fatty acids and VLCFA have been discovered and investigated. Polyunsaturated VLCFA in plants are formed mainly by 20:1 elongation into new monounsaturated acids, which are then imported into chloroplasts, where they are further desaturated. The formation of saturated VLCFA and their further transformation into a number of aliphatic compounds included in cuticular waxes and suberin require the coordinated activity of a large number of different enzymes.
Keywords: cuticular waxes; desaturation of fatty acids; elongase systems; polar lipids; storage lipids; suberin; very-long-chain fatty acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kim J., Jung J.H., Lee S.B., Go Y.S., Kim H.J., Cahoon R., Markham J.E., Cahoon E.B., Suh M.C. Arabidopsis 3-Ketoacyl-coenzyme A synthase9 is involved in the synthesis of tetracosanoic acids as precursors of cuticular waxes, suberins, sphingolipids and phospholipids. Plant Physiol. 2013;162:567–580. doi: 10.1104/pp.112.210450. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

