Treating High COD Dyeing Wastewater via a Regenerative Sorption-Oxidation Process Using a Nano-Pored Activated Carbon
- PMID: 35563142
- PMCID: PMC9104435
- DOI: 10.3390/ijms23094752
Treating High COD Dyeing Wastewater via a Regenerative Sorption-Oxidation Process Using a Nano-Pored Activated Carbon
Abstract
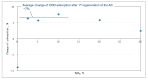
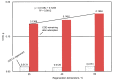
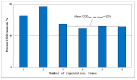
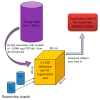
Nowadays, the structural complexity of dyes used in the textile industry and the widely adopted water-saving strategy in the dyeing processes often fail plants' biological wastewater treatment units due to chemical oxygen demand (COD) overload. To alleviate this problems, this study investigated a regenerable adsorption-oxidation process to treat dyeing wastewater with COD around 10,000 mg/dm3 using a highly nano-pored activated carbon (AC) as a COD adsorbent, followed by its regeneration using hydrogen peroxide as an oxidizing reagent. In addition to studying AC's COD adsorption and oxidation performance, its operational treatment conditions in terms of temperature and pH were assessed. The results firstly demonstrated that about 50-60% of the COD was consistently adsorbed during the repeated adsorption operation before reaching AC's maximum adsorption capacity (qmax) of 0.165 g-COD/g-AC. The optimal pH and temperature during adsorption were 4.7 and 25 °C, respectively. Secondly, AC regeneration was accomplished by using an initial peroxide concentration of 2.5% (by wt %) and EDTA-Fe of 2.12 mmole/dm3. The reuse of the regenerated ACs was doable. Surprisingly, after the first AC regeneration, the COD adsorption capacity of the regenerated AC even increased by ~7% with respect to the virgin AC. Thirdly, the results of a five-consecutive adsorption-regeneration operation showed that a total of 0.3625 g COD was removed by the 5 g AC used, which was equivalent to an adsorption capacity (q) of 0.0725 (= 0.3625/5) g-COD/g-AC during each adsorption stage. Based on the obtained results, a regenerable COD adsorption-oxidation process using a nano-pored AC to treat the high-textile-COD wastewater looks promising. Thus, a conceptual treatment unit was proposed, and its potential benefits and limitations were addressed.
Keywords: a regenerable adsorption–oxidation process; activated carbon (AC); adsorption capacity; high COD wastewater; hydrogen peroxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Metwally M.A., Abdel-Galil E., Metwally A., Amer F.A. New azodisperse dyes with thiazole, thiophene, pyridone and pyrazolone moiety for dyeing polyester fabrics. Dye. Pigment. 2012;92:902–908. doi: 10.1016/j.dyepig.2011.07.009. - DOI
-
- Sayed A.Z., Aboul-Fetouh M.S., Nassar H.S. Synthesis, biological activity and dyeing performance of some novel azo disperse dyes incorporating pyrazolo[1,5-a]pyrimidines for dyeing of polyester fabrics. J. Mol. Struct. 2012;1010:146–151. doi: 10.1016/j.molstruc.2011.11.046. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

