Molecular Alterations of the Endocannabinoid System in Psychiatric Disorders
- PMID: 35563156
- PMCID: PMC9104141
- DOI: 10.3390/ijms23094764
Molecular Alterations of the Endocannabinoid System in Psychiatric Disorders
Abstract
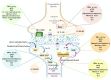
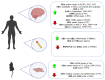
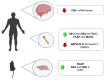
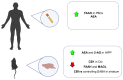
The therapeutic benefits of the current medications for patients with psychiatric disorders contrast with a great variety of adverse effects. The endocannabinoid system (ECS) components have gained high interest as potential new targets for treating psychiatry diseases because of their neuromodulator role, which is essential to understanding the regulation of many brain functions. This article reviewed the molecular alterations in ECS occurring in different psychiatric conditions. The methods used to identify alterations in the ECS were also described. We used a translational approach. The animal models reproducing some behavioral and/or neurochemical aspects of psychiatric disorders and the molecular alterations in clinical studies in post-mortem brain tissue or peripheral tissues were analyzed. This article reviewed the most relevant ECS changes in prevalent psychiatric diseases such as mood disorders, schizophrenia, autism, attentional deficit, eating disorders (ED), and addiction. The review concludes that clinical research studies are urgently needed for two different purposes: (1) To identify alterations of the ECS components potentially useful as new biomarkers relating to a specific disease or condition, and (2) to design new therapeutic targets based on the specific alterations found to improve the pharmacological treatment in psychiatry.
Keywords: endocannabinoid system; method; molecular alteration; psychiatric disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Endocannabinoid System Components as Potential Biomarkers in Psychiatry.Front Psychiatry. 2020 Apr 27;11:315. doi: 10.3389/fpsyt.2020.00315. eCollection 2020. Front Psychiatry. 2020. PMID: 32395111 Free PMC article. Review.
-
Modulation of Endocannabinoid System Components in Depression: Pre-Clinical and Clinical Evidence.Int J Mol Sci. 2022 May 15;23(10):5526. doi: 10.3390/ijms23105526. Int J Mol Sci. 2022. PMID: 35628337 Free PMC article. Review.
-
Molecular Findings Guiding the Modulation of the Endocannabinoid System as a Potential Target to Treat Schizophrenia.Adv Exp Med Biol. 2022;1400:89-103. doi: 10.1007/978-3-030-97182-3_7. Adv Exp Med Biol. 2022. PMID: 35930228
-
Endocannabinoid system dysfunction in mood and related disorders.Acta Psychiatr Scand. 2011 Oct;124(4):250-61. doi: 10.1111/j.1600-0447.2011.01687.x. Epub 2011 Mar 9. Acta Psychiatr Scand. 2011. PMID: 21916860 Review.
-
Biomarkers of the Endocannabinoid System in Substance Use Disorders.Biomolecules. 2022 Mar 3;12(3):396. doi: 10.3390/biom12030396. Biomolecules. 2022. PMID: 35327588 Free PMC article. Review.
Cited by
-
In silico analyses of the involvement of GPR55, CB1R and TRPV1: response to THC, contribution to temporal lobe epilepsy, structural modeling and updated evolution.Front Neuroinform. 2024 Feb 7;18:1294939. doi: 10.3389/fninf.2024.1294939. eCollection 2024. Front Neuroinform. 2024. PMID: 38404644 Free PMC article.
-
Early-life iron deficiency persistently disrupts affective behaviour in mice.Ann Med. 2023 Dec;55(1):1265-1277. doi: 10.1080/07853890.2023.2191003. Ann Med. 2023. PMID: 37096819 Free PMC article.
-
The Role of the Endocannabinoid System in Binge Eating Disorder.Int J Mol Sci. 2023 May 31;24(11):9574. doi: 10.3390/ijms24119574. Int J Mol Sci. 2023. PMID: 37298525 Free PMC article. Review.
-
Management of sleep disorders in autism spectrum disorder with co-occurring attention-deficit hyperactivity disorder: update for clinicians.BJPsych Open. 2023 Dec 13;10(1):e11. doi: 10.1192/bjo.2023.589. BJPsych Open. 2023. PMID: 38088185 Free PMC article. Review.
-
Genetic variation of the 5-HT1A rs6295, 5-HT2A rs6311, and CNR1 rs1049353 and an altered endocannabinoid system in depressed patients.Brain Behav. 2023 Dec;13(12):e3323. doi: 10.1002/brb3.3323. Epub 2023 Nov 20. Brain Behav. 2023. PMID: 37984468 Free PMC article.
References
-
- Cameron C., Watson D., Robinson J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: A retrospective evaluation. J. Clin. Psychopharmacol. 2014;34:559–564. doi: 10.1097/JCP.0000000000000180. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

