Linoleic Acid Attenuates Denervation-Induced Skeletal Muscle Atrophy in Mice through Regulation of Reactive Oxygen Species-Dependent Signaling
- PMID: 35563168
- PMCID: PMC9105847
- DOI: 10.3390/ijms23094778
Linoleic Acid Attenuates Denervation-Induced Skeletal Muscle Atrophy in Mice through Regulation of Reactive Oxygen Species-Dependent Signaling
Abstract
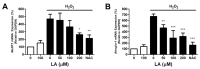
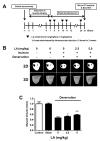
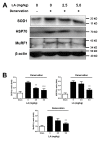
Muscle atrophy is a major muscle disease, the symptoms of which include decreased muscle volume leading to insufficient muscular support during exercise. One cause of muscle atrophy is the induction of oxidative stress by reactive oxygen species (ROS). This study aimed to identify the antioxidant mechanism of linoleic acid (LA) in muscle atrophy caused by oxidative stress. H2O2 has been used to induce oxidative stress in myoblasts in vitro. C2C12 myoblasts treated with H2O2 exhibited decreased viability and increased ROS synthesis. However, with LA treatment, the cells tended to recover from oxidative effects similar to those of the control groups. At the molecular level, the expression of superoxide dismutase 1 (SOD1), Bax, heat shock protein 70 (HSP70), and phosphorylated forkhead box protein O1 was increased by oxidative stress, causing apoptosis. LA treatment suppressed these changes. In addition, the expression of MuRF1 and Atrogin-1/MAFbx mRNA increased under oxidative stress but not in the LA-treated group. Sciatic denervation of C57BL/6 mice manifested as atrophy of the skeletal muscle in micro-computed tomography (micro-CT). The protein expression levels of SOD1, HSP70, and MuRF1 did not differ between the atrophied muscle tissues and C2C12 myoblasts under oxidative stress. With LA treatment, muscle atrophy recovered and protein expression was restored to levels similar to those in the control. Therefore, this study suggests that LA may be a candidate substance for preventing muscle atrophy.
Keywords: antioxidant; linoleic acid; muscle atrophy; oxidative stress; sciatic denervation.
Conflict of interest statement
The authors have declared no conflicting interests.
Figures



Similar articles
-
Anti-skeletal muscle atrophy effect of Oenothera odorata root extract via reactive oxygen species-dependent signaling pathways in cellular and mouse model.Biosci Biotechnol Biochem. 2016;80(1):80-8. doi: 10.1080/09168451.2015.1075861. Epub 2015 Aug 19. Biosci Biotechnol Biochem. 2016. PMID: 26613402
-
The preventive effect of β-carotene on denervation-induced soleus muscle atrophy in mice.Br J Nutr. 2013 Apr 28;109(8):1349-58. doi: 10.1017/S0007114512003297. Epub 2012 Oct 9. Br J Nutr. 2013. PMID: 23046823
-
Targeted ablation of the cellular inhibitor of apoptosis 1 (cIAP1) attenuates denervation-induced skeletal muscle atrophy.Skelet Muscle. 2019 May 24;9(1):13. doi: 10.1186/s13395-019-0201-6. Skelet Muscle. 2019. PMID: 31126323 Free PMC article.
-
The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy.Pflugers Arch. 2011 Mar;461(3):325-35. doi: 10.1007/s00424-010-0919-9. Epub 2011 Jan 11. Pflugers Arch. 2011. PMID: 21221630 Review.
-
Is there a common mechanism linking muscle wasting in various disease types?Curr Opin Support Palliat Care. 2007 Dec;1(4):287-92. doi: 10.1097/SPC.0b013e3282f35238. Curr Opin Support Palliat Care. 2007. PMID: 18685377 Review.
Cited by
-
Gracilaria chorda attenuates obesity-related muscle wasting through activation of SIRT1/PGC1α in skeletal muscle of mice.Food Sci Nutr. 2024 Apr 4;12(7):5077-5086. doi: 10.1002/fsn3.4157. eCollection 2024 Jul. Food Sci Nutr. 2024. PMID: 39055231 Free PMC article.
-
Analysis of nutritional risk, skeletal muscle depletion, and lipid metabolism phenotype in acute radiation enteritis.World J Gastrointest Surg. 2023 Dec 27;15(12):2831-2843. doi: 10.4240/wjgs.v15.i12.2831. World J Gastrointest Surg. 2023. PMID: 38222011 Free PMC article.
-
Plant-Derived Treatments for Different Types of Muscle Atrophy.Phytother Res. 2025 Feb;39(2):1107-1138. doi: 10.1002/ptr.8420. Epub 2025 Jan 2. Phytother Res. 2025. PMID: 39743857 Free PMC article. Review.
-
Carthamus tinctorius L. (Safflower) Flower Extract Attenuates Hepatic Injury and Steatosis in a Rat Model of Type 2 Diabetes Mellitus via Nrf2-Dependent Hypoglycemic, Antioxidant, and Hypolipidemic Effects.Antioxidants (Basel). 2024 Sep 10;13(9):1098. doi: 10.3390/antiox13091098. Antioxidants (Basel). 2024. PMID: 39334757 Free PMC article.
-
Avocado Oil: Recent Advances in Its Anti-diabetic Potential.Curr Med Sci. 2025 Feb;45(1):11-24. doi: 10.1007/s11596-025-00010-w. Epub 2025 Feb 25. Curr Med Sci. 2025. PMID: 39998768 Review.
References
-
- Pompeani N., Rybalka E., Latchman H., Murphy R.M., Croft K., Hayes A. Skeletal muscle atrophy in sedentary Zucker obese rats is not caused by calpain-mediated muscle damage or lipid peroxidation induced by oxidative stress. J. Negat. Results Biomed. 2014;13:19–29. doi: 10.1186/s12952-014-0019-z. - DOI - PMC - PubMed
-
- Cervenakova L., Protas I.I., Hirano A., Votiakov V.I., Nedzved M.K., Kolomiets N.D., Taller I., Park K.Y., Sambuughin N., Gajdusek D.C., et al. Progressive muscular atrophy variant of familial amyotrophic lateral sclerosis (PMA/ALS) J. Neurol. Sci. 2000;177:124–130. doi: 10.1016/S0022-510X(00)00350-6. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

