Ferroptosis and Apoptosis Are Involved in the Formation of L-Selenomethionine-Induced Ocular Defects in Zebrafish Embryos
- PMID: 35563172
- PMCID: PMC9100823
- DOI: 10.3390/ijms23094783
Ferroptosis and Apoptosis Are Involved in the Formation of L-Selenomethionine-Induced Ocular Defects in Zebrafish Embryos
Abstract
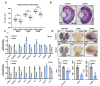
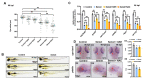
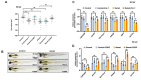
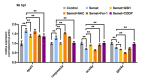
Selenium is an essential trace element for humans and other vertebrates, playing an important role in antioxidant defense, neurobiology and reproduction. However, the toxicity of excessive selenium has not been thoroughly evaluated, especially for the visual system of vertebrates. In this study, fertilized zebrafish embryos were treated with 0.5 µM L-selenomethionine to investigate how excessive selenium alters zebrafish eye development. Selenium-stressed zebrafish embryos showed microphthalmia and altered expression of genes required for retinal neurogenesis. Moreover, ectopic proliferation, disrupted mitochondrial morphology, elevated ROS-induced oxidative stress, apoptosis and ferroptosis were observed in selenium-stressed embryos. Two antioxidants-reduced glutathione (GSH) and N-acetylcysteine (NAC)-and the ferroptosis inhibitor ferrostatin (Fer-1) were unable to rescue selenium-induced eye defects, but the ferroptosis and apoptosis activator cisplatin (CDDP) was able to improve microphthalmia and the expression of retina-specific genes in selenium-stressed embryos. In summary, our results reveal that ferroptosis and apoptosis might play a key role in selenium-induced defects of embryonic eye development. The findings not only provide new insights into selenium-induced cellular damage and death, but also important implications for studying the association between excessive selenium and ocular diseases in the future.
Keywords: L-selenomethionine; ROS; apoptosis; ferroptosis; microphthalmia.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Özkaya D., Nazıroğlu M., Vanyorek L., Muhamad S. Involvement of TRPM2 Channel on Hypoxia-Induced Oxidative Injury, Inflammation, and Cell Death in Retinal Pigment Epithelial Cells: Modulator Action of Selenium Nanoparticles. Biol. Trace Elem. Res. 2021;199:1356–1369. doi: 10.1007/s12011-020-02556-3. - DOI - PubMed
-
- Ananth S., Miyauchi S., Thangaraju M., Jadeja R.N., Bartoli M., Ganapathy V., Martin P.M. Selenomethionine (Se-Met) induces the cystine/glutamate exchanger SLC7A11 in cultured human retinal pigment epithelial (rpe) cells: Implications for antioxidant therapy in aging retina. Antioxidants. 2021;10:9. doi: 10.3390/antiox10010009. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

