ApoA1 Deficiency Reshapes the Phenotypic and Molecular Characteristics of Bone Marrow Adipocytes in Mice
- PMID: 35563223
- PMCID: PMC9100701
- DOI: 10.3390/ijms23094834
ApoA1 Deficiency Reshapes the Phenotypic and Molecular Characteristics of Bone Marrow Adipocytes in Mice
Abstract
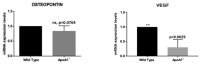
In the present study, we studied the effect of apolipoprotein A-1 (APOA1) on the spatial and molecular characteristics of bone marrow adipocytes, using well-characterized ApoA1 knockout mice. APOA1 is a central regulator of high-density lipoprotein cholesterol (HDL-C) metabolism, and thus HDL; our recent work showed that deficiency of APOA1 increases bone marrow adiposity in mice. We found that ApoA1 deficient mice have greatly elevated adipocytes within their bone marrow compared to wild type counterparts. Morphologically, the increased adipocytes were similar to white adipocytes, and displayed proximal tibial-end localization. Marrow adipocytes from wild type mice were significantly fewer and did not display a bone-end distribution pattern. The mRNA levels of the brown/beige adipocyte-specific markers Ucp1, Dio2, Pat2, and Pgc1a; and the expression of leptin were greatly reduced in the ApoA1 knock-out in comparison to the wild-type mice. In the knock-out mice, adiponectin was remarkably elevated. In keeping with the close ties of hematopoietic stem cells and marrow adipocytes, using flow cytometry we found that the elevated adiposity in the ApoA1 knockout mice is associated with a significant reduction in the compartments of hematopoietic stem cells and common myeloid, but not of the common lymphoid, progenitors. Moreover, the 'beiging'-related marker osteopontin and the angiogenic factor VEGF were also reduced in the ApoA1 knock-out mice, further supporting the notion that APOA1-and most probably HDL-C-regulate bone marrow microenvironment, favoring beige/brown adipocyte characteristics.
Keywords: apolipoprotein A-1; beige (hybrid) adipose tissue; brown adipose tissue; high-density lipoprotein; white adipose tissue.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Adiponectin stimulates Sca1+CD34--adipocyte precursor cells associated with hyperplastic expansion and beiging of brown and white adipose tissue.Metabolism. 2024 Feb;151:155716. doi: 10.1016/j.metabol.2023.155716. Epub 2023 Nov 2. Metabolism. 2024. PMID: 37918793
-
Isoform and tissue dependent impact of apolipoprotein E on adipose tissue metabolic activation: The role of apolipoprotein A1.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Feb;1865(2):158551. doi: 10.1016/j.bbalip.2019.158551. Epub 2019 Oct 31. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 31678510
-
Overexpression of Adiponectin Receptor 1 Inhibits Brown and Beige Adipose Tissue Activity in Mice.Int J Mol Sci. 2021 Jan 18;22(2):906. doi: 10.3390/ijms22020906. Int J Mol Sci. 2021. PMID: 33477525 Free PMC article.
-
Bone marrow adipocytes.Adipocyte. 2017 Jul 3;6(3):193-204. doi: 10.1080/21623945.2017.1367881. Epub 2017 Aug 24. Adipocyte. 2017. PMID: 28872979 Free PMC article. Review.
-
Brown and beige adipose tissue: a novel therapeutic strategy for obesity and type 2 diabetes mellitus.Adipocyte. 2021 Dec;10(1):48-65. doi: 10.1080/21623945.2020.1870060. Adipocyte. 2021. PMID: 33403891 Free PMC article. Review.
Cited by
-
Association of apolipoprotein A1 levels with lumbar bone mineral density and β-CTX in osteoporotic fracture individuals: a cross-sectional investigation.Front Med (Lausanne). 2024 Jul 31;11:1415739. doi: 10.3389/fmed.2024.1415739. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39144661 Free PMC article.
-
The association between the non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio and the risk of osteoporosis among U.S. adults: analysis of NHANES data.Lipids Health Dis. 2024 Jun 3;23(1):161. doi: 10.1186/s12944-024-02152-7. Lipids Health Dis. 2024. PMID: 38831342 Free PMC article.
-
Effects of Different-Syllable Aggressive Calls on Food Intake and Gene Expression in Vespertilio sinensis.Animals (Basel). 2023 Jan 15;13(2):306. doi: 10.3390/ani13020306. Animals (Basel). 2023. PMID: 36670846 Free PMC article.
-
Apolipoprotein A1 is associated with osteocalcin and bone mineral density rather than high-density lipoprotein cholesterol in Chinese postmenopausal women with type 2 diabetes mellitus.Front Med (Lausanne). 2023 Jun 15;10:1182866. doi: 10.3389/fmed.2023.1182866. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37396919 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

