Exosomal miRNAs as a Promising Source of Biomarkers in Colorectal Cancer Progression
- PMID: 35563246
- PMCID: PMC9103063
- DOI: 10.3390/ijms23094855
Exosomal miRNAs as a Promising Source of Biomarkers in Colorectal Cancer Progression
Abstract
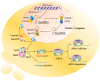
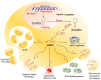
Colorectal cancer (CRC) is the third most common type of cancer worldwide amongst males and females. CRC treatment is multidisciplinary, often including surgery, chemotherapy, and radiotherapy. Early diagnosis of CRC can lead to treatment initiation at an earlier stage. Blood biomarkers are currently used to detect CRC, but because of their low sensitivity and specificity, they are considered inadequate diagnostic tools and are used mainly for following up patients for recurrence. It is necessary to detect novel, noninvasive, specific, and sensitive biomarkers for the screening and diagnosis of CRC at earlier stages. The tumor microenvironment (TME) has an essential role in tumorigenesis; for example, extracellular vesicles (EVs) such as exosomes can play a crucial role in communication between cancer cells and different components of TME, thereby inducing tumor progression. The importance of miRNAs that are sorted into exosomes has recently attracted scientists' attention. Some unique sequences of miRNAs are favorably packaged into exosomes, and it has been illustrated that particular miRNAs can be directed into exosomes by special mechanisms that occur inside the cells. This review illustrates and discusses the sorted and transported exosomal miRNAs in the CRC microenvironment and their impact on CRC progression as well as their potential use as biomarkers.
Keywords: biomarkers; colorectal cancer; exosomes; extracellular vesicles; miRNAs; tumor microenvironment.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

