Inactivation of E. coli, S. aureus, and Bacteriophages in Biofilms by Humidified Air Plasma
- PMID: 35563247
- PMCID: PMC9100691
- DOI: 10.3390/ijms23094856
Inactivation of E. coli, S. aureus, and Bacteriophages in Biofilms by Humidified Air Plasma
Abstract
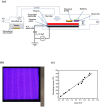
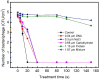
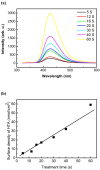
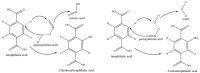
In this study, humidified air dielectric barrier discharge (DBD) plasma was used to inactivate Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), and bacteriophages in biofilms containing DNA, NaCl, carbohydrates, and proteins. The humidified DBD plasma was very effective in the inactivation of microbes in the (≤1.0 μm) biofilms. The number of surviving E. coli, S. aureus, and bacteriophages in the biofilms was strongly dependent on the constituent and thickness of the biofilms and was greatly reduced when the plasma treatment time increased from 5 s to 150 s. Our analysis shows that the UV irradiation was not responsible for the inactivation of microbes in biofilms. The short-lived RONS generated in the humidified air DBD plasma were not directly involved in the inactivation process; however, they recombined or reacted with other species to generate the long-lived RONS. Long-lived RONS diffused into the biofilms to generate very active species, such as ONOOH and OH. This study indicates that the geminated NO2 and OH pair formed due to the homolysis of ONOOH can cause the synergistic oxidation of various organic molecules in the aqueous solution. Proteins in the biofilm were highly resistant to the inactivation of microbes in biofilms, which is presumably due to the existence of the unstable functional groups in the proteins. The unsaturated fatty acids, cysteine-rich proteins, and sulfur-methyl thioether groups in the proteins were easily oxidized by the geminated NO2 and OH pair.
Keywords: E. coli; bacteriophage; biofilms; dielectric barrier discharge; disinfection; humidified air plasma; plasma inactivation; proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Ki S.H., Baik K.Y., Choi E.H. Effects of humidity on room disinfection by dielectric barrier discharge plasma. J. Phys. D Appl. Phys. 2019;42:425204. doi: 10.1088/1361-6463/ab3066. - DOI
-
- Kogelheide F., Voigt F., Hillebrand B., Moeller R., Fiebrandt M. The role of humidity and UV-C emission in the inactivation of B. subtilis spores during atmospheric-pressure dielectric barrier discharge treatment. J. Phys. D Appl. Phys. 2020;29:295201. doi: 10.1088/1361-6463/ab77cc. - DOI
-
- Elena V.S., Aleksandra Y.L., Roman A.L., Elena V.V., Mariam A.A., Svetlana A.E., Aleksey V.S. Bidirectional mass transfer-based generation of plasma-activated water mist with antibacterial properties. Plasma Process. Polym. 2020;17:e2000058.
-
- Moldgy A., Nayak G., Aboubakr H.A., Goyal S.M., Bruggeman P.J. Inactivation of virus and bacteria using cold atmospheric pressure air plasmas and the role of reactive nitrogen species. J. Phys. D Appl. Phys. 2020;43:434004. doi: 10.1088/1361-6463/aba066. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

