Mesenchymal Stem Cells and Extracellular Vesicles Derived from Canine Adipose Tissue Ameliorates Inflammation, Skin Barrier Function and Pruritus by Reducing JAK/STAT Signaling in Atopic Dermatitis
- PMID: 35563259
- PMCID: PMC9101369
- DOI: 10.3390/ijms23094868
Mesenchymal Stem Cells and Extracellular Vesicles Derived from Canine Adipose Tissue Ameliorates Inflammation, Skin Barrier Function and Pruritus by Reducing JAK/STAT Signaling in Atopic Dermatitis
Abstract
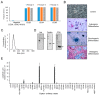
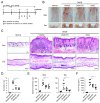
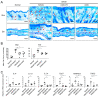
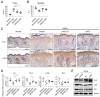
Canine atopic dermatitis (AD) is a common chronic inflammatory skin disorder resulting from imbalance between T lymphocytes. Current canine AD treatments use immunomodulatory drugs, but some of the dogs have limitations that do not respond to standard treatment, or relapse after a period of time. Thus, the purpose of this study was to evaluate the immunomodulatory effect of mesenchymal stem cells derived from canine adipose tissue (cASCs) and cASCs-derived extracellular vesicles (cASC-EVs) on AD. First, we isolated and characterized cASCs and cASCs-EVs to use for the improvement of canine atopic dermatitis. Here, we investigated the effect of cASCs or cASC-EVs on DNCB-induced AD in mice, before using for canine AD. Interestingly, we found that cASCs and cASC-EVs improved AD-like dermatitis, and markedly decreased levels of serum IgE, (49.6%, p = 0.002 and 32.1%, p = 0.016 respectively) epidermal inflammatory cytokines and chemokines, such as IL-4 (32%, p = 0.197 and 44%, p = 0.094 respectively), IL-13 (47.4%, p = 0.163, and 50.0%, p = 0.039 respectively), IL-31 (64.3%, p = 0.030 and 76.2%, p = 0.016 respectively), RANTES (66.7%, p = 0.002 and 55.6%, p = 0.007) and TARC (64%, p = 0.016 and 86%, p = 0.010 respectively). In addition, cASCs or cASC-EVs promoted skin barrier repair by restoring transepidermal water loss, enhancing stratum corneum hydration and upregulating the expression levels of epidermal differentiation proteins. Moreover, cASCs or cASC-EVs reduced IL-31/TRPA1-mediated pruritus and activation of JAK/STAT signaling pathway. Taken together, these results suggest the potential of cASCs or cASC-EVs for the treatment of chronic inflammation and damaged skin barrier in AD or canine AD.
Keywords: atopic dermatitis; canine; extracellular vesicle; inflammation; mesenchymal stem cell; pruritus; skin barrier function.
Conflict of interest statement
S.Y.K. and T.H.Y. are employees of GNG CELL Co., Ltd., R&D Center. T.-W.C. is CEO and stockholder of JIN BioCell Co., Ltd. Sang-Soo Lee who is funder and CEO (founder) of GNG CELL Co., Ltd., had no role in data collection, design, analyses, interpretation of data, manuscript preparation, or publication. All rights is reserved by GNG CELL, although this paper was written by supporting technique and idea through a joint research contract (GNG CELL Co./Kyungpook National University/JIN BioCell Co., Ltd.). No potential conflict of interest was reported by all other authors.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

