Hepatoprotective Effects of Albumin-Encapsulated Nanoparticles of a Curcumin Derivative COP-22 against Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Injury in Mice
- PMID: 35563293
- PMCID: PMC9102161
- DOI: 10.3390/ijms23094903
Hepatoprotective Effects of Albumin-Encapsulated Nanoparticles of a Curcumin Derivative COP-22 against Lipopolysaccharide/D-Galactosamine-Induced Acute Liver Injury in Mice
Abstract
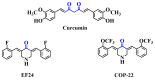
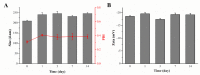
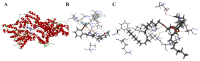
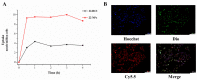
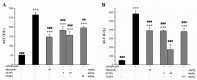
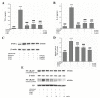
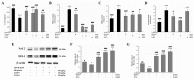
Acute liver injury (ALI) is a severe syndrome and can further develop into acute liver failure (ALF) which can lead to high mortality and cause irreversible liver injuries in the clinic. Liver transplantation is the most common treatment; however, liver donors are lacking, and the progression of ALF is rapid. Nanoparticles can increase the bioavailability and the targeted accumulation of drugs in the liver, so as to significantly improve the therapeutic effect of ALI. Curcumin derivative COP-22 exhibits low cytotoxicity and effective anti-inflammatory activity; however, it has poor water solubility. In this study, COP-22-loaded bovine serum albumin (BSA) nanoparticles (22 NPs) were prepared and characterized. They exhibit effective hepatoprotective effects by inhibiting inflammation, oxidative stress, and apoptosis on Lipopolysaccharide/D-Galactosamine-induced acute liver injury of mice. The anti-inflammatory activity of 22 NPs is related to the regulation of the NF-κB signaling pathways; the antioxidant activity is related to the regulation of the Nrf2 signaling pathways; and the apoptosis activity is related to mitochondrial pathways, involving Bcl-2 family and Caspase-3 protein. These three cellular pathways are interrelated and affected each other. Moreover, 22 NPs could be passively targeted to accumulate in the liver through the retention effect and are more easily absorbed than 22.HCl salt in the liver.
Keywords: acute liver injury; apoptosis; bovine serum albumin nanoparticles; curcumin derivative; inflammation; oxidative stress.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







Similar articles
-
Evaluation of Hepatoprotective Effects of Piperlongumine Derivative PL 1-3-Loaded Albumin Nanoparticles on Lipopolysaccharide/d-Galactosamine-Induced Acute Liver Injury in Mice.Mol Pharm. 2022 Dec 5;19(12):4576-4587. doi: 10.1021/acs.molpharmaceut.2c00215. Epub 2022 Aug 16. Mol Pharm. 2022. PMID: 35971845
-
Hepatoprotective effects and structure-activity relationship of five flavonoids against lipopolysaccharide/d-galactosamine induced acute liver failure in mice.Int Immunopharmacol. 2019 Mar;68:171-178. doi: 10.1016/j.intimp.2018.12.059. Epub 2019 Jan 11. Int Immunopharmacol. 2019. PMID: 30641432
-
Protective Role of 4-Octyl Itaconate in Murine LPS/D-GalN-Induced Acute Liver Failure via Inhibiting Inflammation, Oxidative Stress, and Apoptosis.Oxid Med Cell Longev. 2021 Aug 17;2021:9932099. doi: 10.1155/2021/9932099. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34457120 Free PMC article.
-
Preclinical validation of silibinin/albumin nanoparticles as an applicable system against acute liver injury.Acta Biomater. 2022 Jul 1;146:385-395. doi: 10.1016/j.actbio.2022.04.021. Epub 2022 Apr 20. Acta Biomater. 2022. PMID: 35460909
-
Propofol attenuates inflammatory response and apoptosis to protect d-galactosamine/lipopolysaccharide induced acute liver injury via regulating TLR4/NF-κB/NLRP3 pathway.Int Immunopharmacol. 2019 Dec;77:105974. doi: 10.1016/j.intimp.2019.105974. Epub 2019 Nov 15. Int Immunopharmacol. 2019. PMID: 31735662
Cited by
-
Natural Products for Acetaminophen-Induced Acute Liver Injury: A Review.Molecules. 2023 Dec 1;28(23):7901. doi: 10.3390/molecules28237901. Molecules. 2023. PMID: 38067630 Free PMC article. Review.
-
COP-22 Alleviates D-Galactose-Induced Brain Aging by Attenuating Oxidative Stress, Inflammation, and Apoptosis in Mice.Mol Neurobiol. 2024 Sep;61(9):6708-6720. doi: 10.1007/s12035-024-03976-1. Epub 2024 Feb 12. Mol Neurobiol. 2024. PMID: 38347285 Free PMC article.
-
Domino hepatocyte transplantation using explanted human livers with metabolic defects attenuates D-GalN/LPS-induced acute liver failure.J Transl Med. 2022 Oct 20;20(1):479. doi: 10.1186/s12967-022-03674-3. J Transl Med. 2022. PMID: 36266691 Free PMC article.
-
Oxidative Stress, Inflammation, Gut Dysbiosis: What Can Polyphenols Do in Inflammatory Bowel Disease?Antioxidants (Basel). 2023 Apr 20;12(4):967. doi: 10.3390/antiox12040967. Antioxidants (Basel). 2023. PMID: 37107341 Free PMC article. Review.
-
Apoptosis and cell cycle arrest of bone marrow cells by green-synthesized silver but not albumin nanoparticles.Toxicol Rep. 2025 Feb 13;14:101960. doi: 10.1016/j.toxrep.2025.101960. eCollection 2025 Jun. Toxicol Rep. 2025. PMID: 40026477 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

