Modulation of Mismatch Repair and the SOCS1/p53 Axis by microRNA-155 in the Colon of Patients with Primary Sclerosing Cholangitis
- PMID: 35563301
- PMCID: PMC9100906
- DOI: 10.3390/ijms23094905
Modulation of Mismatch Repair and the SOCS1/p53 Axis by microRNA-155 in the Colon of Patients with Primary Sclerosing Cholangitis
Abstract
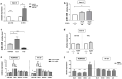
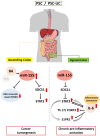
Deficient mismatch repair (MMR) proteins may lead to DNA damage and microsatellite instability. Primary sclerosing cholangitis (PSC) is a risk factor for colitis-associated colon cancer. MiR-155 is suggested to act as a key regulating node, linking inflammation and tumorigenesis. However, its involvement in the chronic colitis of PSC-UC patients has not been examined. We investigated the involvement of miR-155 in the dysregulation of MMR genes and colitis in PSC patients. Colon tissue biopsies were obtained from patients with PSC, PSC with concomitant ulcerative colitis (PSC-UC), uncomplicated UC, and healthy controls (n = 10 per group). In the ascending colon of PSC and PSC-UC patients, upregulated miR-155 promoted high microsatellite instability and induced signal transducer and activator of transcription 3 (STAT-3) expression via the inhibition of suppressors of cytokine signalling 1 (SOCS1). In contrast, the absence of miR-155 overexpression in the sigmoid colon of PSC-UC patients activated the Il-6/S1PR1 signalling pathway and imbalanced the IL17/FOXP3 ratio, which reinforces chronic colitis. Functional studies on human intestinal epithelial cells (HT-29 and NCM460D) confirmed the role of miR-155 over-expression in the inhibition of MMR genes and the modulation of p53. Moreover, those cells produced more TNFα upon a lipopolysaccharide challenge, which led to the suppression of miR-155. Additionally, exposure to bile acids induced upregulation of miR-155 in Caco-2 cell lines. Thus, under different conditions, miR-155 is involved in either neoplastic transformation in the ascending colon or chronic colitis in the sigmoid colon of patients with PSC. New insight into local modulation of microRNAs, that may alter the course of the disease, could be used for further research on potential therapeutic applications.
Keywords: biomarker; colorectal cancer; inflammation; miR-155; microsatellite instability; primary sclerosing cholangitis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

