At Embryo Implantation Site IL-35 Secreted by Trophoblast, Polarizing T Cells towards IL-35+ IL-10+ IL-4+ Th2-Type Cells, Could Favour Fetal Allograft Tolerance and Pregnancy Success
- PMID: 35563316
- PMCID: PMC9103079
- DOI: 10.3390/ijms23094926
At Embryo Implantation Site IL-35 Secreted by Trophoblast, Polarizing T Cells towards IL-35+ IL-10+ IL-4+ Th2-Type Cells, Could Favour Fetal Allograft Tolerance and Pregnancy Success
Abstract
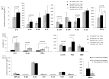
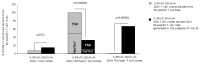
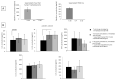
We investigated the role of rhIL-35, at low concentrations compatible with those produced by human trophoblast cells (less than 1 ng/mL), on human T helper (Th) cell functions and the presence of decidual IL-35-producing Th cells in human pregnancy. We found that human trophoblast cells produced IL-35 but not IL-4 or IL-10. RhIL-35, at concentrations produced by human trophoblasts, polarized T cells towards IL-35+, IL-10+, IL-4+ Th2-type cells and to Foxp3+ EBI3+ p35+ T reg cells producing IL-35 but not IL-10 and IL-4. Moreover, rhIL-35 at low concentrations did not suppress the proliferation of Th cells but stimulated IL-4 and IL-10 production by established Th clones. In particular, Th1-type clones acquired the capacity to produce IL-4. In addition, purified human trophoblast cell supernatants containing IL-35 upregulated IL-4 and IL-10 production by Th clones. Finally, IL-35+, IL-10+, IL-4+ Th2-type cells, which were found to be induced by low concentrations of IL-35 compatible with those produced by human trophoblasts, are exclusively present in the decidua of a successful pregnancy and at the embryo implantation site, suggesting their stringent dependence on trophoblast cells. Thus, the proximity of Th cells to IL-35-producing trophoblasts could be the determining factor for the differentiation of IL-35+, IL-10+, IL-4+ Th2-type cells that are crucial for human pregnancy success.
Keywords: IL-10; IL-35; IL-4; Th2 cells; Treg cells; ectopic pregnancy; implantation; p35; pregnancy; trophoblast.
Conflict of interest statement
The authors declare no commercial or financial conflict of interest.
Figures




References
-
- Yuan X., Paez-Cortez J., Schmitt-Knosalla I., D’Addio F., Mfarrej B., Donnarumma M., Habicht A., Clarkson M.R., Iacomini J., Glimcher L.H., et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J. Exp. Med. 2008;205:3133–3144. doi: 10.1084/jem.20081937. - DOI - PMC - PubMed
-
- Burns W.R., Wang Y., Tang P.C., Ranjbaran H., Iakimov A., Kim J., Cuffy M., Bai Y., Pober J.S., Tellides G. Recruitment of CXCR3+ and CCR5+ T cells and production of interferon-gamma-inducible chemokines in rejecting human arteries. Am. J. Transplant. 2005;5:1226–1236. doi: 10.1111/j.1600-6143.2005.00892.x. - DOI - PubMed
-
- Waaga A.M., Gasser M., Kist-van Holthe J.E., Najafian N., Müller A., Vella J.P., Womer K.L., Chandraker A., Khoury S.J., Sayegh M.H. Regulatory functions of self-restricted MHC class II allopeptide-specific Th2 clones in vivo. J. Clin. Investig. 2001;107:909–916. doi: 10.1172/JCI11427. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

