Transduction of Brain Neurons in Juvenile Chum Salmon (Oncorhynchus keta) with Recombinant Adeno-Associated Hippocampal Virus Injected into the Cerebellum during Long-Term Monitoring
- PMID: 35563338
- PMCID: PMC9101580
- DOI: 10.3390/ijms23094947
Transduction of Brain Neurons in Juvenile Chum Salmon (Oncorhynchus keta) with Recombinant Adeno-Associated Hippocampal Virus Injected into the Cerebellum during Long-Term Monitoring
Abstract
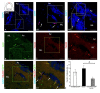
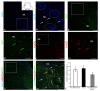
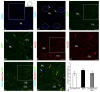
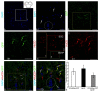
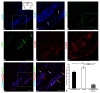
Corpus cerebelli in juvenile chum salmon is a multiprojective region of the brain connected via afferent and efferent projections with the higher regions of the brainstem and synencephalon, as well as with multiprojection regions of the medulla oblongata and spinal cord. During the postembryonic development of the cerebellum in chum salmon, Oncorhynchus keta, the lateral part of the juvenile cerebellum gives rise to the caudomedial part of the definitive cerebellum, which is consistent with the data reported for zebrafish and mouse cerebellum. Thus, the topographic organization of the cerebellum and its efferents are similar between fish (chum salmon and zebrafish) and mammals, including mice and humans. The distributions of recombinant adeno-associated viral vectors (rAAVs) after an injection of the base vector into the cerebellum have shown highly specific patterns of transgene expression in bipolar neurons in the latero-caudal lobe of the juvenile chum tectum opticum. The distribution of rAAVs in the dorsal thalamus, epithalamus, nucleus rotundus, and pretectal complex indicates the targeted distribution of the transgene via the thalamo-cerebellar projections. The detection of GFP expression in the cells of the epiphysis and posterior tubercle of juvenile chum salmon is associated with the transgene's distribution and with the cerebrospinal fluid flow, the brain ventricles and its outer surface. The direct delivery of the rAAV into the central nervous system by intracerebroventricular administration allows it to spread widely in the brain. Thus, the presence of special projection areas in the juvenile chum salmon cerebellum, as well as outside it, and the identification of the transgene's expression in them confirm the potential ability of rAAVs to distribute in both intracerebellar and afferent and efferent extracerebellar projections of the cerebellum.
Keywords: Purkinje cells; cerebellum; cerebrospinal fluid; chum salmon; eurydendroid cells; genetically encoded calcium indicators; green fluorescent protein; intracerebroventricular administration; recombinant adeno-associated viruses; thalamo-cerebellar projections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
A Confocal Microscopic Study of Gene Transfer into the Mesencephalic Tegmentum of Juvenile Chum Salmon, Oncorhynchus keta, Using Mouse Adeno-Associated Viral Vectors.Int J Mol Sci. 2021 May 26;22(11):5661. doi: 10.3390/ijms22115661. Int J Mol Sci. 2021. PMID: 34073457 Free PMC article.
-
Ultrastructural Study and Immunohistochemical Characteristics of Mesencephalic Tegmentum in Juvenile Chum Salmon (Oncorhynchus keta) Brain After Acute Traumatic Injury.Int J Mol Sci. 2025 Jan 14;26(2):644. doi: 10.3390/ijms26020644. Int J Mol Sci. 2025. PMID: 39859360 Free PMC article.
-
Mechanical Brain Injury Increases Cells' Production of Cystathionine β-Synthase and Glutamine Synthetase, but Reduces Pax2 Expression in the Telencephalon of Juvenile Chum Salmon, Oncorhynchus keta.Int J Mol Sci. 2021 Jan 28;22(3):1279. doi: 10.3390/ijms22031279. Int J Mol Sci. 2021. PMID: 33525421 Free PMC article.
-
Post-Traumatic Expressions of Aromatase B, Glutamine Synthetase, and Cystathionine-Beta-Synthase in the Cerebellum of Juvenile Chum Salmon, Oncorhynchus keta.Int J Mol Sci. 2024 Mar 14;25(6):3299. doi: 10.3390/ijms25063299. Int J Mol Sci. 2024. PMID: 38542274 Free PMC article.
-
Development, circuitry, and function of the zebrafish cerebellum.Cell Mol Life Sci. 2023 Jul 25;80(8):227. doi: 10.1007/s00018-023-04879-5. Cell Mol Life Sci. 2023. PMID: 37490159 Free PMC article. Review.
Cited by
-
Exploring cerebellar transcranial magnetic stimulation in post-stroke limb dysfunction rehabilitation: a narrative review.Front Neurosci. 2025 Feb 3;19:1405637. doi: 10.3389/fnins.2025.1405637. eCollection 2025. Front Neurosci. 2025. PMID: 39963260 Free PMC article. Review.
-
Adult Neurogenesis of Teleost Fish Determines High Neuronal Plasticity and Regeneration.Int J Mol Sci. 2024 Mar 25;25(7):3658. doi: 10.3390/ijms25073658. Int J Mol Sci. 2024. PMID: 38612470 Free PMC article. Review.
References
-
- Lewis T.B., Glasgow J.N., Glandon A.M., Curiel D.T., Standaert D.G. Transduction of brain dopamine neurons by adenoviral vectors is modulated by CAR expression: Rationale for tropism modified vectors in PD gene therapy. PLoS ONE. 2010;5:e12672. doi: 10.1371/journal.pone.0012672. - DOI - PMC - PubMed
-
- Jan A., Richner M., Vægter C.B., Nyengaard J.R., Jensen P.H. Gene transfer in rodent nervous tissue following hindlimb intramuscular delivery of recombinant adeno-associated virus serotypes AAV2/6, AAV2/8, and AAV2/9. Neurosci. Insights. 2019;14:1179069519889022. doi: 10.1177/1179069519889022. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

