Novel Antibacterial Copolymers Based on Quaternary Ammonium Urethane-Dimethacrylate Analogues and Triethylene Glycol Dimethacrylate
- PMID: 35563344
- PMCID: PMC9103508
- DOI: 10.3390/ijms23094954
Novel Antibacterial Copolymers Based on Quaternary Ammonium Urethane-Dimethacrylate Analogues and Triethylene Glycol Dimethacrylate
Abstract
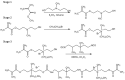
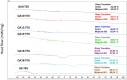
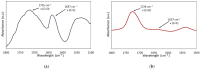
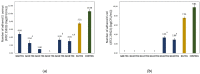
The growing scale of secondary caries and occurrence of antibiotic-resistant bacterial strains require the development of antibacterial dental composites. It can be achieved by the chemical introduction of quaternary ammonium dimethacrylates into dental composites. In this study, physicochemical and antibacterial properties of six novel copolymers consisting of 60 wt. % quaternary ammonium urethane-dimethacrylate analogues (QAUDMA) and 40 wt. % triethylene glycol dimethacrylate (TEGDMA) were investigated. Uncured compositions had suitable refractive index (RI), density (dm), and glass transition temperature (Tgm). Copolymers had low polymerization shrinkage (S), high degree of conversion (DC) and high glass transition temperature (Tgp). They also showed high antibacterial effectiveness against S. aureus and E. coli bacterial strains. It was manifested by the reduction in cell proliferation, decrease in the number of bacteria adhered on their surfaces, and presence of growth inhibition zones. It can be concluded that the copolymerization of bioactive QAUDMAs with TEGDMA provided copolymers with high antibacterial activity and rewarding physicochemical properties.
Keywords: antibacterial activity; photocurable copolymers; physicochemical properties; quaternary ammonium compounds; urethane-dimethacrylates.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures






References
-
- Bowen R.L. Dental Filling Material Comprising Vinyl Silane Treated Fused Silica and a Binder Consisting of the Reaction Product of Bis Phenol and Glycidyl Acrylate. 3,066,112A. U.S. Patent. 1692 June 26;
-
- Watts D.C. Adhesives and Sealants. In: Ratner B., Hoffman A., Schoen F., Lemons J., editors. Biomaterials Science: An Introduction to Materials. 3rd ed. Elsevier Inc.; Amsterdam, The Netherlands: 2013. pp. 889–904.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

