Impact of Cerebral Amyloid Angiopathy in Two Transgenic Mouse Models of Cerebral β-Amyloidosis: A Neuropathological Study
- PMID: 35563362
- PMCID: PMC9103818
- DOI: 10.3390/ijms23094972
Impact of Cerebral Amyloid Angiopathy in Two Transgenic Mouse Models of Cerebral β-Amyloidosis: A Neuropathological Study
Abstract
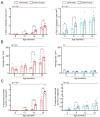
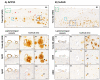
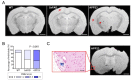
The pathological accumulation of parenchymal and vascular amyloid-beta (Aβ) are the main hallmarks of Alzheimer's disease (AD) and Cerebral Amyloid Angiopathy (CAA), respectively. Emerging evidence raises an important contribution of vascular dysfunction in AD pathology that could partially explain the failure of anti-Aβ therapies in this field. Transgenic mice models of cerebral β-amyloidosis are essential to a better understanding of the mechanisms underlying amyloid accumulation in the cerebrovasculature and its interactions with neuritic plaque deposition. Here, our main objective was to evaluate the progression of both parenchymal and vascular deposition in APP23 and 5xFAD transgenic mice in relation to age and sex. We first showed a significant age-dependent accumulation of extracellular Aβ deposits in both transgenic models, with a greater increase in APP23 females. We confirmed that CAA pathology was more prominent in the APP23 mice, demonstrating a higher progression of Aβ-positive vessels with age, but not linked to sex, and detecting a pronounced burden of cerebral microbleeds (cMBs) by magnetic resonance imaging (MRI). In contrast, 5xFAD mice did not present CAA, as shown by the negligible Aβ presence in cerebral vessels and the occurrence of occasional cMBs comparable to WT mice. In conclusion, the APP23 mouse model is an interesting tool to study the overlap between vascular and parenchymal Aβ deposition and to evaluate future disease-modifying therapy before its translation to the clinic.
Keywords: 5xFAD; APP23; cerebral microbleeds; cerebral β-amyloidosis; preclinical MRI.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Murine versus human apolipoprotein E4: differential facilitation of and co-localization in cerebral amyloid angiopathy and amyloid plaques in APP transgenic mouse models.Acta Neuropathol Commun. 2015 Nov 10;3:70. doi: 10.1186/s40478-015-0250-y. Acta Neuropathol Commun. 2015. PMID: 26556230 Free PMC article.
-
Efficient Seeding of Cerebral Vascular Aβ-Amyloidosis by Recombinant AβM1-42 Amyloid Fibrils.J Mol Biol. 2025 Feb 1;437(3):168923. doi: 10.1016/j.jmb.2024.168923. Epub 2024 Dec 24. J Mol Biol. 2025. PMID: 39725269
-
Early-onset formation of parenchymal plaque amyloid abrogates cerebral microvascular amyloid accumulation in transgenic mice.J Biol Chem. 2014 Jun 20;289(25):17895-908. doi: 10.1074/jbc.M113.536565. Epub 2014 May 14. J Biol Chem. 2014. PMID: 24828504 Free PMC article.
-
Hereditary and sporadic forms of abeta-cerebrovascular amyloidosis and relevant transgenic mouse models.Int J Mol Sci. 2009 Apr 23;10(4):1872-1895. doi: 10.3390/ijms10041872. Int J Mol Sci. 2009. PMID: 19468344 Free PMC article. Review.
-
Cerebral amyloid angiopathy and Alzheimer disease - one peptide, two pathways.Nat Rev Neurol. 2020 Jan;16(1):30-42. doi: 10.1038/s41582-019-0281-2. Epub 2019 Dec 11. Nat Rev Neurol. 2020. PMID: 31827267 Free PMC article. Review.
Cited by
-
Deletion of Murine APP Aggravates Tau and Amyloid Pathologies in the 5xFADXTg30 Alzheimer's Disease Model.Biomolecules. 2025 Jan 21;15(2):159. doi: 10.3390/biom15020159. Biomolecules. 2025. PMID: 40001463 Free PMC article.
-
Cognitive decline, Aβ pathology, and blood-brain barrier function in aged 5xFAD mice.Fluids Barriers CNS. 2024 Mar 27;21(1):29. doi: 10.1186/s12987-024-00531-x. Fluids Barriers CNS. 2024. PMID: 38532486 Free PMC article.
-
Hippocampal glial inflammatory markers are differentially altered in a novel mouse model of perimenopausal cerebral amyloid angiopathy.Front Aging Neurosci. 2023 Nov 15;15:1280218. doi: 10.3389/fnagi.2023.1280218. eCollection 2023. Front Aging Neurosci. 2023. PMID: 38035277 Free PMC article.
-
The Roles of the Amyloid Beta Monomers in Physiological and Pathological Conditions.Biomedicines. 2023 May 10;11(5):1411. doi: 10.3390/biomedicines11051411. Biomedicines. 2023. PMID: 37239082 Free PMC article. Review.
-
Animal models of Alzheimer's disease: Current strategies and new directions.Zool Res. 2024 Nov 18;45(6):1385-1407. doi: 10.24272/j.issn.2095-8137.2024.274. Zool Res. 2024. PMID: 39572020 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

