The SCO2102 Protein Harbouring a DnaA II Protein-Interaction Domain Is Essential for the SCO2103 Methylenetetrahydrofolate Reductase Positioning at Streptomyces Sporulating Hyphae, Enhancing DNA Replication during Sporulation
- PMID: 35563376
- PMCID: PMC9099993
- DOI: 10.3390/ijms23094984
The SCO2102 Protein Harbouring a DnaA II Protein-Interaction Domain Is Essential for the SCO2103 Methylenetetrahydrofolate Reductase Positioning at Streptomyces Sporulating Hyphae, Enhancing DNA Replication during Sporulation
Abstract
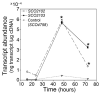
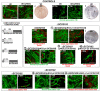
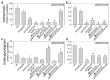
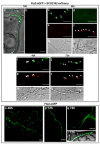
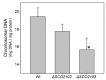
Streptomyces DNA replication starts with the DnaA binding to the origin of replication. Differently to most bacteria, cytokinesis only occurs during sporulation. Cytokinesis is modulated by the divisome, an orderly succession of proteins initiated by FtsZ. Here, we characterised SCO2102, a protein harbouring a DnaA II protein-protein interaction domain highly conserved in Streptomyces. The ΔSCO2102 knockout shows highly delayed sporulation. SCO2102-mCherry frequently co-localises with FtsZ-eGFP during sporulation and greatly reduces FtsZ-eGFP Z-ladder formation, suggesting a role of SCO2102 in sporulation. SCO2102 localises up-stream of SCO2103, a methylenetetrahydrofolate reductase involved in methionine and dTMP synthesis. SCO2102/SCO2103 expression is highly regulated, involving two promoters and a conditional transcription terminator. The ΔSCO2103 knockout shows reduced DNA synthesis and a non-sporulating phenotype. SCO2102-mCherry co-localises with SCO2103-eGFP during sporulation, and SCO2102 is essential for the SCO2103 positioning at sporulating hyphae, since SCO2103-eGFP fluorescent spots are absent in the ΔSCO2102 knockout. We propose a model in which SCO2102 positions SCO2103 in sporulating hyphae, facilitating nucleotide biosynthesis for chromosomal replication. To the best of our knowledge, SCO2102 is the first protein harbouring a DnaA II domain specifically found during sporulation, whereas SCO2103 is the first methylenetetrahydrofolate reductase found to be essential for Streptomyces sporulation.
Keywords: DnaA; SCO2102 DnaA homologue; SCO2103 MTHFR; Streptomyces; cell division; differentiation; sporulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Hopwood D.A. Streptomyces in Nature and Medicine: The Antibiotic Makers. Oxford University Press; New York, NY, USA: Oxford, UK: 2007.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

