SIRT2 Is Critical for Sheep Oocyte Maturation through Regulating Function of Surrounding Granulosa Cells
- PMID: 35563403
- PMCID: PMC9104768
- DOI: 10.3390/ijms23095013
SIRT2 Is Critical for Sheep Oocyte Maturation through Regulating Function of Surrounding Granulosa Cells
Abstract
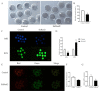
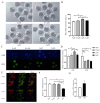
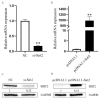
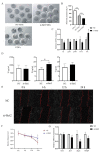
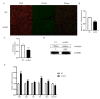
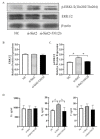
Oocyte in vitro maturation is crucial for in vitro embryo production technology, which provides oocytes resources for in vitro fertilization and somatic cell nuclear transfer. Previous studies proved that SIRT2, a member of the sirtuin family, plays a role in oocyte meiosis, but its role in sheep oocyte maturation and its regulating mechanism remains unknown. Firstly, we confirmed the role of Sirt2 in sheep oocytes maturation by supplementation of SIRT2 inhibitor and activator. To further explore the specific mechanism, we performed knockdown of Sirt2 in granulosa cells and then cocultured them with oocytes. Moreover, we determined the effects of Sirt2 on granulosa cell oxidative apoptosis, cell migration, and diffusion, and examined its effects on granulosa cell mitochondrial function, mitophagy, and steroid hormone levels. The results showed that supplementation of SIRT2 inhibitor decreased the oocytes maturation rate (69.28% ± 1.28 vs. 45.74% ± 4.74, p < 0.05), while resveratrol, a SIRT2 activator, increased its maturation rate (67.44% ± 1.68 vs. 78.52 ± 1.28, p < 0.05). Knockdown of Sirt2 in sheep granulosa cells also reduced the oocytes maturation rate (47.98% ± 1.43 vs. 33.60% ± 1.77, p < 0.05), and led to decreased cell migration and expansion ability, oxidative apoptosis, abnormal mitochondrial gene expression, decreased mitochondrial membrane potential and ATP level, and increased mitophagy level. Overexpression of Sirt2 improved mitochondrial membrane potential and ATP level and improved mitochondrial function. Furthermore, we found that Sirt2 knockdown in granulosa cells promotes the secretion of P4 through regulating p-ERK1/2. In conclusion the present study showed that SIRT2 is critical for sheep oocyte maturation through regulating the function of ovarian granulosa cells, especially affecting its mitochondrial function.
Keywords: SIRT2; mitochondria; mitophagy; oocytes in vitro maturation; sheep granulosa cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Li H.J., Sutton-McDowall M.L., Wang X., Sugimura S., Thompson J.G., Gilchrist R.B. Extending prematuration with cAMP modulators enhances the cumulus contribution to oocyte antioxidant defence and oocyte quality via gap junctions. Hum. Reprod. 2016;31:810–821. doi: 10.1093/humrep/dew020. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 2021YFD1200401/National Key R&D Program of China
- C2019204260/Natural Science Foundation of Hebei Province of China
- ZD2020108/Key Project of Educational Commission of Hebei Province of China
- YJ2021013/Special Project for Talents Enrollment of Hebei Agricultural University
- KY2021006/Basic Research Funds for Colleges of Hebei province
LinkOut - more resources
Full Text Sources
Miscellaneous

