Actin Stress Fibers Response and Adaptation under Stretch
- PMID: 35563485
- PMCID: PMC9101353
- DOI: 10.3390/ijms23095095
Actin Stress Fibers Response and Adaptation under Stretch
Abstract
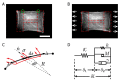
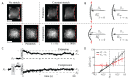
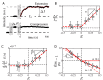
One of the many effects of soft tissues under mechanical solicitation in the cellular damage produced by highly localized strain. Here, we study the response of peripheral stress fibers (SFs) to external stretch in mammalian cells, plated onto deformable micropatterned substrates. A local fluorescence analysis reveals that an adaptation response is observed at the vicinity of the focal adhesion sites (FAs) due to its mechanosensor function. The response depends on the type of mechanical stress, from a Maxwell-type material in compression to a complex scenario in extension, where a mechanotransduction and a self-healing process takes place in order to prevent the induced severing of the SF. A model is proposed to take into account the effect of the applied stretch on the mechanics of the SF, from which relevant parameters of the healing process are obtained. In contrast, the repair of the actin bundle occurs at the weak point of the SF and depends on the amount of applied strain. As a result, the SFs display strain-softening features due to the incorporation of new actin material into the bundle. In contrast, the response under compression shows a reorganization with a constant actin material suggesting a gliding process of the SFs by the myosin II motors.
Keywords: SF response; actin stress fibers; cell mechanics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Mechanosensitive myosin II but not cofilin primarily contributes to cyclic cell stretch-induced selective disassembly of actin stress fibers.Am J Physiol Cell Physiol. 2021 Jun 1;320(6):C1153-C1163. doi: 10.1152/ajpcell.00225.2020. Epub 2021 Apr 21. Am J Physiol Cell Physiol. 2021. PMID: 33881935
-
Cyclic tensile strain controls cell shape and directs actin stress fiber formation and focal adhesion alignment in spreading cells.PLoS One. 2013 Oct 28;8(10):e77328. doi: 10.1371/journal.pone.0077328. eCollection 2013. PLoS One. 2013. PMID: 24204809 Free PMC article.
-
Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization.J Cell Sci. 2009 May 15;122(Pt 10):1665-79. doi: 10.1242/jcs.042986. Epub 2009 Apr 28. J Cell Sci. 2009. PMID: 19401336
-
The inner workings of stress fibers - from contractile machinery to focal adhesions and back.J Cell Sci. 2016 Apr 1;129(7):1293-304. doi: 10.1242/jcs.180927. J Cell Sci. 2016. PMID: 27037413 Review.
-
LIM proteins in actin cytoskeleton mechanoresponse.Trends Cell Biol. 2014 Oct;24(10):575-83. doi: 10.1016/j.tcb.2014.04.009. Epub 2014 Jun 2. Trends Cell Biol. 2014. PMID: 24933506 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

