Relationship between Neuronal Damage/Death and Astrogliosis in the Cerebral Motor Cortex of Gerbil Models of Mild and Severe Ischemia and Reperfusion Injury
- PMID: 35563487
- PMCID: PMC9100252
- DOI: 10.3390/ijms23095096
Relationship between Neuronal Damage/Death and Astrogliosis in the Cerebral Motor Cortex of Gerbil Models of Mild and Severe Ischemia and Reperfusion Injury
Abstract
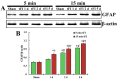
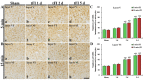
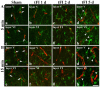
Neuronal loss (death) occurs selectively in vulnerable brain regions after ischemic insults. Astrogliosis is accompanied by neuronal death. It can change the molecular expression and morphology of astrocytes following ischemic insults. However, little is known about cerebral ischemia and reperfusion injury that can variously lead to damage of astrocytes according to the degree of ischemic injury, which is related to neuronal damage/death. Thus, the purpose of this study was to examine the relationship between damage to cortical neurons and astrocytes using gerbil models of mild and severe transient forebrain ischemia induced by blocking the blood supply to the forebrain for five or 15 min. Significant ischemia tFI-induced neuronal death occurred in the deep layers (layers V and VI) of the motor cortex: neuronal death occurred earlier and more severely in gerbils with severe ischemia than in gerbils with mild ischemia. Distinct astrogliosis was detected in layers V and VI. It gradually increased with time after both ischemiae. The astrogliosis was significantly higher in severe ischemia than in mild ischemia. The ischemia-induced increase of glial fibrillary acidic protein (GFAP; a maker of astrocyte) expression in severe ischemia was significantly higher than that in mild ischemia. However, GFAP-immunoreactive astrocytes were apparently damaged two days after both ischemiae. At five days after ischemiae, astrocyte endfeet around capillary endothelial cells were severely ruptured. They were more severely ruptured by severe ischemia than by mild ischemia. However, the number of astrocytes stained with S100 was significantly higher in severe ischemia than in mild ischemia. These results indicate that the degree of astrogliosis, including the disruption (loss) of astrocyte endfeet following ischemia and reperfusion in the forebrain, might depend on the severity of ischemia and that the degree of ischemia-induced neuronal damage may be associated with the degree of astrogliosis.
Keywords: S100; astrocyte endfeet; blood–brain barrier; cortical layer; glial fibrillary acidic protein; ischemia and reperfusion injury.
Conflict of interest statement
The authors have declared that there is no financial conflict of interest.
Figures





Similar articles
-
Neuronal damage and gliosis in the somatosensory cortex induced by various durations of transient cerebral ischemia in gerbils.Brain Res. 2013 May 13;1510:78-88. doi: 10.1016/j.brainres.2013.03.008. Epub 2013 Mar 23. Brain Res. 2013. PMID: 23528266
-
Changes in the level of glial fibrillary acidic protein (GFAP) after mild and severe focal cerebral ischemia.Chin J Physiol. 1999 Dec 31;42(4):227-35. Chin J Physiol. 1999. PMID: 10707898
-
Astrocyte-Derived Estrogen Regulates Reactive Astrogliosis and is Neuroprotective following Ischemic Brain Injury.J Neurosci. 2020 Dec 9;40(50):9751-9771. doi: 10.1523/JNEUROSCI.0888-20.2020. Epub 2020 Nov 6. J Neurosci. 2020. PMID: 33158962 Free PMC article.
-
Astrocyte-mediated mechanisms contribute to traumatic brain injury pathology.WIREs Mech Dis. 2023 Sep-Oct;15(5):e1622. doi: 10.1002/wsbm.1622. Epub 2023 Jun 18. WIREs Mech Dis. 2023. PMID: 37332001 Free PMC article. Review.
-
Glial function (and dysfunction) in the normal & ischemic brain.Neuropharmacology. 2018 May 15;134(Pt B):218-225. doi: 10.1016/j.neuropharm.2017.11.009. Epub 2017 Nov 6. Neuropharmacology. 2018. PMID: 29122627 Free PMC article. Review.
Cited by
-
Trans- and Cis-Phosphorylated Tau Protein: New Pieces of the Puzzle in the Development of Neurofibrillary Tangles in Post-Ischemic Brain Neurodegeneration of the Alzheimer's Disease-like Type.Int J Mol Sci. 2024 Mar 7;25(6):3091. doi: 10.3390/ijms25063091. Int J Mol Sci. 2024. PMID: 38542064 Free PMC article.
-
Factors Contributing to Resistance to Ischemia-Reperfusion Injury in Olfactory Mitral Cells.Int J Mol Sci. 2025 May 25;26(11):5079. doi: 10.3390/ijms26115079. Int J Mol Sci. 2025. PMID: 40507890 Free PMC article. Review.
-
Therapeutic Hypothermia after Cardiac Arrest Attenuates Hindlimb Paralysis and Damage of Spinal Motor Neurons and Astrocytes through Modulating Nrf2/HO-1 Signaling Pathway in Rats.Cells. 2023 Jan 26;12(3):414. doi: 10.3390/cells12030414. Cells. 2023. PMID: 36766758 Free PMC article.
-
PFKFB3 ameliorates ischemia-induced neuronal damage by reducing reactive oxygen species and inhibiting nuclear translocation of Cdk5.Sci Rep. 2024 Oct 21;14(1):24694. doi: 10.1038/s41598-024-75031-x. Sci Rep. 2024. PMID: 39433564 Free PMC article.
-
Acetylated Tau Protein: A New Piece in the Puzzle between Brain Ischemia and Alzheimer's Disease.Int J Mol Sci. 2022 Aug 16;23(16):9174. doi: 10.3390/ijms23169174. Int J Mol Sci. 2022. PMID: 36012440 Free PMC article.
References
-
- Kondo T., Yoshida S., Nagai H., Takeshita A., Mino M., Morioka H., Nakajima T., Kusakabe K.T., Okada T. Transient forebrain ischemia induces impairment in cognitive performance prior to extensive neuronal cell death in mongolian gerbil (meriones unguiculatus) J. Vet. Sci. 2018;19:505–511. doi: 10.4142/jvs.2018.19.4.505. - DOI - PMC - PubMed
-
- Ahn J.H., Song M., Kim H., Lee T.K., Park C.W., Park Y.E., Lee J.C., Cho J.H., Kim Y.M., Hwang I.K., et al. Differential regional infarction, neuronal loss and gliosis in the gerbil cerebral hemisphere following 30 min of unilateral common carotid artery occlusion. Metab. Brain Dis. 2019;34:223–233. doi: 10.1007/s11011-018-0345-9. - DOI - PubMed
-
- de Araujo F.L., Bertolino G., Goncalves R.B., Marini Lde C., Coimbra N.C., de Araujo J.E. Neuropathology and behavioral impairments after three types of global ischemia surgery in meriones unguiculatus: Evidence in motor cortex, hippocampal ca1 region and the neostriatum. J. Neurol. Sci. 2012;312:73–78. doi: 10.1016/j.jns.2011.08.019. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

