NMR Relaxometry Accessing the Relaxation Spectrum in Molecular Glass Formers
- PMID: 35563506
- PMCID: PMC9105706
- DOI: 10.3390/ijms23095118
NMR Relaxometry Accessing the Relaxation Spectrum in Molecular Glass Formers
Abstract
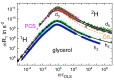
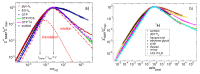
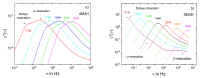
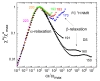
It is a longstanding question whether universality or specificity characterize the molecular dynamics underlying the glass transition of liquids. In particular, there is an ongoing debate to what degree the shape of dynamical susceptibilities is common to various molecular glass formers. Traditionally, results from dielectric spectroscopy and light scattering have dominated the discussion. Here, we show that nuclear magnetic resonance (NMR), primarily field-cycling relaxometry, has evolved into a valuable method, which provides access to both translational and rotational motions, depending on the probe nucleus. A comparison of 1H NMR results indicates that translation is more retarded with respect to rotation for liquids with fully established hydrogen-bond networks; however, the effect is not related to the slow Debye process of, for example, monohydroxy alcohols. As for the reorientation dynamics, the NMR susceptibilities of the structural (α) relaxation usually resemble those of light scattering, while the dielectric spectra of especially polar liquids have a different broadening, likely due to contributions from cross correlations between different molecules. Moreover, NMR relaxometry confirms that the excess wing on the high-frequency flank of the α-process is a generic relaxation feature of liquids approaching the glass transition. However, the relevance of this feature generally differs between various methods, possibly because of their different sensitivities to small-amplitude motions. As a major advantage, NMR is isotope specific; hence, it enables selective studies on a particular molecular entity or a particular component of a liquid mixture. Exploiting these possibilities, we show that the characteristic Cole-Davidson shape of the α-relaxation is retained in various ionic liquids and salt solutions, but the width parameter may differ for the components. In contrast, the low-frequency flank of the α-relaxation can be notably broadened for liquids in nanoscopic confinements. This effect also occurs in liquid mixtures with a prominent dynamical disparity in their components.
Keywords: dielectric spectroscopy; glass transition; ionic and confined liquids; molecular; nuclear magnetic resonance relaxometry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Kudlik A., Benkhof S., Blochowicz T., Tschirwitz C., Rössler E. The dielectric response of simple organic glass formers. J. Mol. Struct. 1999;479:201–218. doi: 10.1016/S0022-2860(98)00871-0. - DOI
-
- Lunkenheimer P., Schneider U., Brand R., Loidl A. Glassy Dynamics. Contemp. Phys. 2000;41:15–36. doi: 10.1080/001075100181259. - DOI
-
- Blochowicz T., Brodin A., Rössler E.A. In: Advances in Chemical Physics. Fractals, Diffusion, and Relaxation in Disordered Complex Systems. Coffey W.T., Kalmykov Y.P., editors. Vol. 133. John Wiley & Sons, Inc.; Hoboken, NJ, USA: 2006. pp. 127–256.
-
- Cummins H.Z., Li G., Hwang Y.H., Shen G.Q., Du W.M., Hernandez J., Tao N.J. Dynamics of supercooled liquids and glasses: Comparison of experiments with theoretical predictions. Z. Phys. B. 1997;103:501–519. doi: 10.1007/s002570050405. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

