Endohedral Gd-Containing Fullerenol: Toxicity, Antioxidant Activity, and Regulation of Reactive Oxygen Species in Cellular and Enzymatic Systems
- PMID: 35563539
- PMCID: PMC9106034
- DOI: 10.3390/ijms23095152
Endohedral Gd-Containing Fullerenol: Toxicity, Antioxidant Activity, and Regulation of Reactive Oxygen Species in Cellular and Enzymatic Systems
Abstract
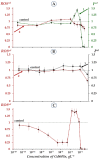
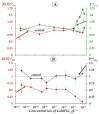
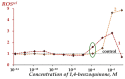
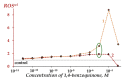
The Gd-containing metallofullerene derivatives are perspective magnetic resonance imaging contrast agents. We studied the bioeffects of a water-soluble fullerene derivative, gadolinium-endohedral fullerenol, with 40−42 oxygen groups (Gd@Fln). Bioluminescent cellular and enzymatic assays were applied to monitor toxicity and antioxidant activity of Gd@Fln in model solutions; bioluminescence was applied as a signaling physiological parameter. The Gd@Fln inhibited bioluminescence at high concentrations (>2·10−1 gL−1), revealing lower toxicity as compared to the previously studied fullerenols. Efficient activation of bioluminescence (up to almost 100%) and consumption of reactive oxygen species (ROS) in bacterial suspension were observed under low-concentration exposure to Gd@Fln (10−3−2·10−1 gL−1). Antioxidant capability of Gd@Fln was studied under conditions of model oxidative stress (i.e., solutions of model organic and inorganic oxidizers); antioxidant coefficients of Gd@Fln were determined at different concentrations and times of exposure. Contents of ROS were evaluated and correlations with toxicity/antioxidant coefficients were determined. The bioeffects of Gd@Fln were explained by hydrophobic interactions, electron affinity, and disturbing of ROS balance in the bioluminescence systems. The results contribute to understanding the molecular mechanism of “hormetic” cellular responses. Advantages of the bioluminescence assays to compare bioeffects of fullerenols based on their structural characteristics were demonstrated.
Keywords: antioxidant activity; bioluminescence bioassay; endohedral fullerenol; gadolinium; hormesis; oxidative stress; reactive oxygen species; toxicity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Effects of Endohedral Gd-Containing Fullerenols with a Different Number of Oxygen Substituents on Bacterial Bioluminescence.Int J Mol Sci. 2024 Jan 5;25(2):708. doi: 10.3390/ijms25020708. Int J Mol Sci. 2024. PMID: 38255785 Free PMC article.
-
Antioxidant Activity and Toxicity of Fullerenols via Bioluminescence Signaling: Role of Oxygen Substituents.Int J Mol Sci. 2019 May 10;20(9):2324. doi: 10.3390/ijms20092324. Int J Mol Sci. 2019. PMID: 31083407 Free PMC article.
-
Toxicity and Antioxidant Activity of Fullerenol C60,70 with Low Number of Oxygen Substituents.Int J Mol Sci. 2021 Jun 15;22(12):6382. doi: 10.3390/ijms22126382. Int J Mol Sci. 2021. PMID: 34203700 Free PMC article.
-
Fullerenol nanoparticles: toxicity and antioxidant activity.Methods Mol Biol. 2013;1028:75-100. doi: 10.1007/978-1-62703-475-3_5. Methods Mol Biol. 2013. PMID: 23740114 Review.
-
Mechanism of ROS scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ROS associated degenerative disorders.Biochim Biophys Acta Gen Subj. 2017 Apr;1861(4):802-813. doi: 10.1016/j.bbagen.2017.01.018. Epub 2017 Jan 20. Biochim Biophys Acta Gen Subj. 2017. PMID: 28115205 Review.
Cited by
-
Effects of Endohedral Gd-Containing Fullerenols with a Different Number of Oxygen Substituents on Bacterial Bioluminescence.Int J Mol Sci. 2024 Jan 5;25(2):708. doi: 10.3390/ijms25020708. Int J Mol Sci. 2024. PMID: 38255785 Free PMC article.
-
Fullerenol C60(OH)36 Protects the Antioxidant Enzymes in Human Erythrocytes against Oxidative Damage Induced by High-Energy Electrons.Int J Mol Sci. 2022 Sep 19;23(18):10939. doi: 10.3390/ijms231810939. Int J Mol Sci. 2022. PMID: 36142851 Free PMC article.
-
Fullerenol C60(OH)36: Antioxidant, Cytoprotective, Anti-Influenza Virus Activity, and Self-Assembly in Aqueous Solutions and Cell Culture Media.Antioxidants (Basel). 2024 Dec 13;13(12):1525. doi: 10.3390/antiox13121525. Antioxidants (Basel). 2024. PMID: 39765853 Free PMC article.
-
Functionalized Magnetite Nanoparticles: Characterization, Bioeffects, and Role of Reactive Oxygen Species in Unicellular and Enzymatic Systems.Int J Mol Sci. 2023 Jan 6;24(2):1133. doi: 10.3390/ijms24021133. Int J Mol Sci. 2023. PMID: 36674650 Free PMC article.
-
Biomedical Application Prospects of Gadolinium Oxide Nanoparticles for Regenerative Medicine.Pharmaceutics. 2024 Dec 23;16(12):1627. doi: 10.3390/pharmaceutics16121627. Pharmaceutics. 2024. PMID: 39771605 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

