Discovery of Raf Family Is a Milestone in Deciphering the Ras-Mediated Intracellular Signaling Pathway
- PMID: 35563547
- PMCID: PMC9101324
- DOI: 10.3390/ijms23095158
Discovery of Raf Family Is a Milestone in Deciphering the Ras-Mediated Intracellular Signaling Pathway
Abstract
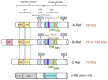
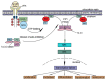
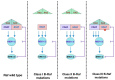
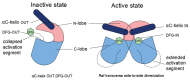
The Ras-Raf-MEK-ERK signaling pathway, the first well-established MAPK pathway, plays essential roles in cell proliferation, survival, differentiation and development. It is activated in over 40% of human cancers owing to mutations of Ras, membrane receptor tyrosine kinases and other oncogenes. The Raf family consists of three isoforms, A-Raf, B-Raf and C-Raf. Since the first discovery of a truncated mutant of C-Raf as a transforming oncogene carried by a murine retrovirus, forty years of extensive studies have provided a wealth of information on the mechanisms underlying the activation, regulation and biological functions of the Raf family. However, the mechanisms by which activation of A-Raf and C-Raf is accomplished are still not completely understood. In contrast, B-Raf can be easily activated by binding of Ras-GTP, followed by cis-autophosphorylation of the activation loop, which accounts for the fact that this isoform is frequently mutated in many cancers, especially melanoma. The identification of oncogenic B-Raf mutations has led to accelerated drug development that targets Raf signaling in cancer. However, the effort has not proved as effective as anticipated, inasmuch as the mechanism of Raf activation involves multiple steps, factors and phosphorylation of different sites, as well as complex interactions between Raf isoforms. In this review, we will focus on the physiological complexity of the regulation of Raf kinases and their connection to the ERK phosphorylation cascade and then discuss the role of Raf in tumorigenesis and the clinical application of Raf inhibitors in the treatment of cancer.
Keywords: ERK; MEK; Raf; growth control; oncogenes; phosphorylation; signal transduction.
Conflict of interest statement
We hereby declare that all authors have no conflict of interest in this work, including affiliations, financial relationships, personal relationships or funding sources that could be perceived as influencing an author’s objectivity regarding the manuscript content (2014).
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

