Is Infantile Hemangioma a Neuroendocrine Tumor?
- PMID: 35563552
- PMCID: PMC9104933
- DOI: 10.3390/ijms23095140
Is Infantile Hemangioma a Neuroendocrine Tumor?
Abstract
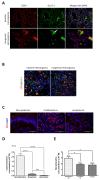
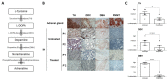
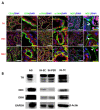
Infantile hemangioma (IH) is the most common infantile tumor, affecting 5-10% of newborns. Propranolol, a nonselective β-adrenergic receptor (ADRB) antagonist, is currently the first-line treatment for severe IH; however, both its mechanism of action and its main cellular target remain poorly understood. Since betablockers can antagonize the effect of natural ADRB agonists, we postulated that the catecholamine produced in situ in IH may have a role in the propranolol response. By quantifying catecholamines in the IH tissues, we found a higher amount of noradrenaline (NA) in untreated proliferative IHs than in involuted IHs or propranolol-treated IHs. We further found that the first three enzymes of the catecholamine biosynthesis pathway are expressed by IH cells and that their levels are reduced in propranolol-treated tumors. To study the role of NA in the pathophysiology of IH and its response to propranolol, we performed an in vitro angiogenesis assay in which IH-derived endothelial cells, pericytes and/or telocytes were incorporated. The results showed that the total tube formation is sensitive to propranolol only when exogenous NA is added in the three-cell model. We conclude that the IH's sensitivity to propranolol depends on crosstalk between the endothelial cells, pericytes and telocytes in the context of a high local amount of local NA.
Keywords: adrenergic; betablocker; catecholamines; hemangioma; propranolol; telocytes.
Conflict of interest statement
A patent has been granted for the use of beta-blockers in infantile capillary hemangiomas with A.T. and C.L.L. as the inventors and Bordeaux University and Bordeaux University Hospital as owners of the patent. None of the authors has any other financial interest related to this work.
Figures


References
-
- Haggstrom A.N., Drolet B.A., Baselga E., Chamlin S.L., Garzon M.C., Horii K.A., Lucky A.W., Mancini A.J., Metry D.W., Newell B., et al. Prospective study of infantile hemangiomas: Clinical characteristics predicting complications and treatment. Pediatrics. 2006;118:882–887. doi: 10.1542/peds.2006-0413. - DOI - PubMed
-
- Léauté-Labrèze C., Hoeger P., Mazereeuw-Hautier J., Guibaud L., Baselga E., Posiunas G., Phillips R.J., Caceres H., Lopez Gutierrez J.C., Ballona R., et al. A Randomized, Controlled Trial of Oral Propranolol in Infantile Hemangioma. N. Engl. J. Med. 2015;372:735–746. doi: 10.1056/NEJMoa1404710. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

