VI-116, A Novel Potent Inhibitor of VRAC with Minimal Effect on ANO1
- PMID: 35563558
- PMCID: PMC9103758
- DOI: 10.3390/ijms23095168
VI-116, A Novel Potent Inhibitor of VRAC with Minimal Effect on ANO1
Abstract
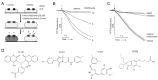
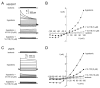
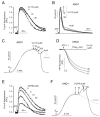
Volume-regulated anion channel (VRAC) is ubiquitously expressed and plays a pivotal role in vertebrate cell volume regulation. A heterologous complex of leucine-rich repeat containing 8A (LRRC8A) and LRRC8B-E constitutes the VRAC, which is involved in various processes such as cell proliferation, migration, differentiation, intercellular communication, and apoptosis. However, the lack of a potent and selective inhibitor of VRAC limits VRAC-related physiological and pathophysiological studies, and most previous VRAC inhibitors strongly blocked the calcium-activated chloride channel, anoctamin 1 (ANO1). In the present study, we performed a cell-based screening for the identification of potent and selective VRAC inhibitors. Screening of 55,000 drug-like small-molecules and subsequent chemical modification revealed 3,3'-((2-hydroxy-3-methoxyphenyl)methylene)bis(4-hydroxy-2H-chromen-2-one) (VI-116), a novel potent inhibitor of VRAC. VI-116 fully inhibited VRAC-mediated I- quenching with an IC50 of 1.27 ± 0.18 μM in LN215 cells and potently blocked endogenous VRAC activity in PC3, HT29 and HeLa cells in a dose-dependent manner. Notably, VI-116 had no effect on intracellular calcium signaling up to 10 μM, which completely inhibited VRAC, and showed high selectivity for VRAC compared to ANO1 and ANO2. However, DCPIB, a VRAC inhibitor, significantly affected ATP-induced increases in intracellular calcium levels and Eact-induced ANO1 activation. In addition, VI-116 showed minimal effect on hERG K+ channel activity up to 10 μM. These results indicate that VI-116 is a potent and selective VRAC inhibitor and a useful research tool for pharmacological dissection of VRAC.
Keywords: ANO1; ANO2; VI-116; VRAC; inhibitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Relationship between TMEM16A/anoctamin 1 and LRRC8A.Pflugers Arch. 2016 Oct;468(10):1751-63. doi: 10.1007/s00424-016-1862-1. Epub 2016 Aug 12. Pflugers Arch. 2016. PMID: 27514381
-
LRRCA8A and ANO1 contribute to serum-induced VRAC in a Ca2+-dependent manners.J Pharmacol Sci. 2020 Jul;143(3):176-181. doi: 10.1016/j.jphs.2020.04.003. Epub 2020 Apr 9. J Pharmacol Sci. 2020. PMID: 32386905
-
Ani9, A Novel Potent Small-Molecule ANO1 Inhibitor with Negligible Effect on ANO2.PLoS One. 2016 May 24;11(5):e0155771. doi: 10.1371/journal.pone.0155771. eCollection 2016. PLoS One. 2016. PMID: 27219012 Free PMC article.
-
Role of volume-regulated and calcium-activated anion channels in cell volume homeostasis, cancer and drug resistance.Channels (Austin). 2015;9(6):380-96. doi: 10.1080/19336950.2015.1089007. Epub 2015 Nov 16. Channels (Austin). 2015. PMID: 26569161 Free PMC article. Review.
-
Molecular Biology and Physiology of Volume-Regulated Anion Channel (VRAC).Curr Top Membr. 2018;81:177-203. doi: 10.1016/bs.ctm.2018.07.005. Epub 2018 Aug 14. Curr Top Membr. 2018. PMID: 30243432 Free PMC article. Review.
Cited by
-
Pharmacological modulation of chloride channels as a therapeutic strategy for neurological disorders.Front Physiol. 2023 Mar 2;14:1122444. doi: 10.3389/fphys.2023.1122444. eCollection 2023. Front Physiol. 2023. PMID: 36935741 Free PMC article. Review.
-
Synthetic Strategies for Improving Solubility: Optimization of Novel Pyrazolo[1,5-a]pyrimidine CFTR Activator That Ameliorates Dry Eye Disease.J Med Chem. 2023 Jan 12;66(1):413-434. doi: 10.1021/acs.jmedchem.2c01382. Epub 2022 Dec 26. J Med Chem. 2023. PMID: 36573286 Free PMC article.
-
ATP-releasing SWELL1 channel in spinal microglia contributes to neuropathic pain.Sci Adv. 2023 Mar 29;9(13):eade9931. doi: 10.1126/sciadv.ade9931. Epub 2023 Mar 29. Sci Adv. 2023. PMID: 36989353 Free PMC article.
-
Upregulation of LRRC8A in the anterior cingulate cortex mediates chronic visceral pain in adult male mice with neonatal maternal deprivation.Mol Pain. 2025 Jan-Dec;21:17448069251324645. doi: 10.1177/17448069251324645. Epub 2025 Feb 17. Mol Pain. 2025. PMID: 39962353 Free PMC article.
-
Inhibition of the LRRC8A channel promotes microglia/macrophage phagocytosis and improves outcomes after intracerebral hemorrhagic stroke.iScience. 2022 Nov 13;25(12):105527. doi: 10.1016/j.isci.2022.105527. eCollection 2022 Dec 22. iScience. 2022. PMID: 36465125 Free PMC article.
References
-
- Voss F.K., Ullrich F., Munch J., Lazarow K., Lutter D., Mah N., Andrade-Navarro M.A., von Kries J.P., Stauber T., Jentsch T.J. Identification of lrrc8 heteromers as an essential component of the volume-regulated anion channel vrac. Science. 2014;344:634–638. doi: 10.1126/science.1252826. - DOI - PubMed
-
- Planells-Cases R., Lutter D., Guyader C., Gerhards N.M., Ullrich F., Elger D.A., Kucukosmanoglu A., Xu G., Voss F.K., Reincke S.M., et al. Subunit composition of vrac channels determines substrate specificity and cellular resistance to pt-based anti-cancer drugs. EMBO J. 2015;34:2993–3008. doi: 10.15252/embj.201592409. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

