Genetic Deficiency of Indoleamine 2,3-dioxygenase Aggravates Vascular but Not Liver Disease in a Nonalcoholic Steatohepatitis and Atherosclerosis Comorbidity Model
- PMID: 35563591
- PMCID: PMC9099704
- DOI: 10.3390/ijms23095203
Genetic Deficiency of Indoleamine 2,3-dioxygenase Aggravates Vascular but Not Liver Disease in a Nonalcoholic Steatohepatitis and Atherosclerosis Comorbidity Model
Abstract
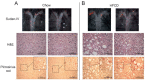
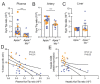
Nonalcoholic steatohepatitis (NASH) is a chronic liver disease that increases cardiovascular disease risk. Indoleamine 2,3-dioxygenase-1 (IDO1)-mediated tryptophan (Trp) metabolism has been proposed to play an immunomodulatory role in several diseases. The potential of IDO1 to be a link between NASH and cardiovascular disease has never been investigated. Using Apoe-/-and Apoe-/-Ido1-/- mice that were fed a high-fat, high-cholesterol diet (HFCD) to simultaneously induce NASH and atherosclerosis, we found that Ido1 deficiency significantly accelerated atherosclerosis after 7 weeks. Surprisingly, Apoe-/-Ido1-/- mice did not present a more aggressive NASH phenotype, including hepatic lipid deposition, release of liver enzymes, and histopathological parameters. As expected, a lower L-kynurenine/Trp (Kyn/Trp) ratio was found in the plasma and arteries of Apoe-/-Ido1-/- mice compared to controls. However, no difference in the hepatic Kyn/Trp ratio was found between the groups. Hepatic transcript analyses revealed that HFCD induced a temporal increase in tryptophan 2,3-dioxygenase (Tdo2) mRNA, indicating an alternative manner to maintain Trp degradation during NASH development in both Apoe-/- and Apoe-/-Ido1-/mice-. Using HepG2 hepatoma cell and THP1 macrophage cultures, we found that iron, TDO2, and Trp degradation may act as important mediators of cross-communication between hepatocytes and macrophages regulating liver inflammation. In conclusion, we show that Ido1 deficiency aggravates atherosclerosis, but not liver disease, in a newly established NASH and atherosclerosis comorbidity model. Our data indicate that the overexpression of TDO2 is an important mechanism that helps in balancing the kynurenine pathway and inflammation in the liver, but not in the artery wall, which likely determined disease outcome in these two target tissues.
Keywords: IDO; NASH; atherosclerosis; immunometabolism; inflammation.
Conflict of interest statement
I.K. reports personal fees from Orion Pharma unrelated to the submitted work. The remaining authors declare no conflict of interest.
Figures



Similar articles
-
Characterization of indoleamine-2,3-dioxygenase 1, tryptophan-2,3-dioxygenase, and Ido1/Tdo2 knockout mice.Toxicol Appl Pharmacol. 2020 Nov 1;406:115216. doi: 10.1016/j.taap.2020.115216. Epub 2020 Aug 29. Toxicol Appl Pharmacol. 2020. PMID: 32871117
-
Indoleamine 2,3-Dioxygenase 1 Deletion-Mediated Kynurenine Insufficiency in Vascular Smooth Muscle Cells Exacerbates Arterial Calcification.Circulation. 2022 Jun 14;145(24):1784-1798. doi: 10.1161/CIRCULATIONAHA.121.057868. Epub 2022 May 18. Circulation. 2022. PMID: 35582948 Free PMC article.
-
Inhibition of indoleamine 2,3-dioxygenase promotes vascular inflammation and increases atherosclerosis in Apoe-/- mice.Cardiovasc Res. 2015 May 1;106(2):295-302. doi: 10.1093/cvr/cvv100. Epub 2015 Mar 5. Cardiovasc Res. 2015. PMID: 25750192
-
Immuno-Metabolic Modulation of Liver Oncogenesis by the Tryptophan Metabolism.Cells. 2021 Dec 9;10(12):3469. doi: 10.3390/cells10123469. Cells. 2021. PMID: 34943977 Free PMC article. Review.
-
Tryptophan Metabolism in Obesity: The Indoleamine 2,3-Dioxygenase-1 Activity and Therapeutic Options.Adv Exp Med Biol. 2024;1460:629-655. doi: 10.1007/978-3-031-63657-8_21. Adv Exp Med Biol. 2024. PMID: 39287867 Review.
Cited by
-
TDO2 deficiency attenuates the hepatic lipid deposition and liver fibrosis in mice with diet-induced non-alcoholic fatty liver disease.Heliyon. 2023 Nov 18;9(11):e22464. doi: 10.1016/j.heliyon.2023.e22464. eCollection 2023 Nov. Heliyon. 2023. PMID: 38074859 Free PMC article.
-
Genome-Wide Profiling of H3K27ac Identifies TDO2 as a Pivotal Therapeutic Target in Metabolic Associated Steatohepatitis Liver Disease.Adv Sci (Weinh). 2024 Dec;11(45):e2404224. doi: 10.1002/advs.202404224. Epub 2024 Oct 4. Adv Sci (Weinh). 2024. PMID: 39364706 Free PMC article.
-
Tryptophan-2,3-Dioxygenase as a Therapeutic Target in Digestive System Diseases.Biology (Basel). 2025 Mar 15;14(3):295. doi: 10.3390/biology14030295. Biology (Basel). 2025. PMID: 40136551 Free PMC article. Review.
-
Indoleamine 2,3-dioxygenase 1-mediated iron metabolism in macrophages contributes to lipid deposition in nonalcoholic steatohepatitis.J Gastroenterol. 2024 Apr;59(4):342-356. doi: 10.1007/s00535-024-02082-2. Epub 2024 Feb 24. J Gastroenterol. 2024. PMID: 38402297
-
Cystathionine gamma-lyase (CTH) inhibition attenuates glioblastoma formation.Redox Biol. 2023 Aug;64:102773. doi: 10.1016/j.redox.2023.102773. Epub 2023 Jun 5. Redox Biol. 2023. PMID: 37300955 Free PMC article.
References
-
- World Health Organization . Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks. World Health Organization; Geneva, Switzerland: 2009.
-
- Cholesterol Treatment Trialists, Collaborators. Mihaylova B., Emberson J., Blackwell L., Keech A., Simes J., Barnes E.H., Voysey M., Gray A., Collins R., et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. - DOI - PMC - PubMed
-
- Tunon J., Back M., Badimon L., Bochaton-Piallat M.L., Cariou B., Daemen M.J., Egido J., Evans P.C., Francis S.E., Ketelhuth D.F., et al. Interplay between hypercholesterolaemia and inflammation in atherosclerosis: Translating experimental targets into clinical practice. Eur. J. Prev. Cardiol. 2018;25:948–955. doi: 10.1177/2047487318773384. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

