The Nitrogen Atom of Vitamin B6 Is Essential for the Catalysis of Radical Aminomutases
- PMID: 35563602
- PMCID: PMC9105233
- DOI: 10.3390/ijms23095210
The Nitrogen Atom of Vitamin B6 Is Essential for the Catalysis of Radical Aminomutases
Abstract
Radical aminomutases are pyridoxal 5'-phosphate (PLP, a B6 vitamer)-dependent enzymes that require the generation of a 5'-deoxyadenosyl radical to initiate the catalytic cycle, to perform a 1,2 amino group shift reaction. The role of the nitrogen atom of PLP in radical aminomutases has not been investigated extensively yet. We report an alternative synthetic procedure to provide easy access to 1-deazaPLP (dAPLP), an isosteric analog of PLP which acts as a probe for studying the role of the nitrogen atom. Our results revealed that lysine 5,6-aminomutase (5,6-LAM), a radical aminomutase, reconstituted with dAPLP cannot turn over a substrate, demonstrating that the nitrogen atom is essential for radical aminomutases. In contrast, biochemical and spectroscopic studies on the S238A variant reconstituted with PLP revealed a minuscule loss of activity. This apparent anomaly can be explained by a water-mediated rescue of activity in S238A, as if mimicking the active site of lysine 2,3-aminomutase. This study leads to a better comprehension of how enzymes harness the optimum capability of PLP to realize catalysis.
Keywords: DFT; EPR; PLP; aminomutase; coenzyme B12; dAdoCbl; mutagenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
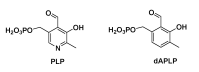






Similar articles
-
Large-scale domain motions and pyridoxal-5'-phosphate assisted radical catalysis in coenzyme B12-dependent aminomutases.Int J Mol Sci. 2014 Feb 20;15(2):3064-87. doi: 10.3390/ijms15023064. Int J Mol Sci. 2014. PMID: 24562332 Free PMC article. Review.
-
Identification of lysine 346 as a functionally important residue for pyridoxal 5'-phosphate binding and catalysis in lysine 2, 3-aminomutase from Bacillus subtilis.Biochemistry. 2001 Jan 16;40(2):596-602. doi: 10.1021/bi002265w. Biochemistry. 2001. PMID: 11148055
-
Pyridoxal-5'-phosphate as the catalyst for radical isomerization in reactions of PLP-dependent aminomutases.Biochim Biophys Acta. 2011 Nov;1814(11):1548-57. doi: 10.1016/j.bbapap.2011.03.005. Epub 2011 Mar 22. Biochim Biophys Acta. 2011. PMID: 21435400 Review.
-
Exploring the mechanism of action of lysine 5,6-aminomutase using EPR and ENDOR spectroscopies.Methods Enzymol. 2022;669:197-228. doi: 10.1016/bs.mie.2021.12.021. Epub 2022 Jan 30. Methods Enzymol. 2022. PMID: 35644172
-
Enzyme catalysis of 1,2-amino shifts: the cooperative action of B6, B12, and aminomutases.J Am Chem Soc. 2001 Sep 12;123(36):8678-89. doi: 10.1021/ja010211j. J Am Chem Soc. 2001. PMID: 11535072
References
-
- Stryer L. Biochemistry. 4th ed. W. H. Freeman and Company; New York, NY, USA: 1995.
-
- Lai R.-Y., Huang S., Fenwick M.K., Hazra A., Zhang Y., Rajashankar K., Philmus B., Kinsland C., Sanders J.M., Ealick S.E., et al. Thiamin Pyrimidine Biosynthesis in Candida albicans: A Remarkable Reaction between Histidine and Pyridoxal Phosphate. J. Am. Chem. Soc. 2012;134:9157–9159. doi: 10.1021/ja302474a. - DOI - PMC - PubMed
-
- Caulkins B.G., Bastin B., Yang C., Neubauer T.J., Young R.P., Hilario E., Huang Y.-M.M., Chang C.-E.A., Fan L., Dunn M.F., et al. Protonation States of the Tryptophan Synthase Internal Aldimine Active Site from Solid-State NMR Spectroscopy: Direct Observation of the Protonated Schiff Base Linkage to Pyridoxal-5′-Phosphate. J. Am. Chem. Soc. 2014;136:12824–12827. doi: 10.1021/ja506267d. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

