Incretin Response to Mixed Meal Challenge in Active Cushing's Disease and after Pasireotide Therapy
- PMID: 35563608
- PMCID: PMC9105040
- DOI: 10.3390/ijms23095217
Incretin Response to Mixed Meal Challenge in Active Cushing's Disease and after Pasireotide Therapy
Abstract
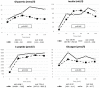
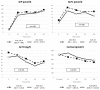
Cushing’s disease (CD) causes diabetes mellitus (DM) through different mechanisms in a significant proportion of patients. Glucose metabolism has rarely been assessed with appropriate testing in CD; we aimed to evaluate hormonal response to a mixed meal tolerance test (MMTT) in CD patients and analyzed the effect of pasireotide (PAS) on glucose homeostasis. To assess gastro-entero-pancreatic hormones response in diabetic (DM+) and non-diabetic (DM−) patients, 26 patients with CD underwent an MMTT. Ten patients were submitted to a second MMTT after two months of PAS 600 µg twice daily. The DM+ group had significantly higher BMI, waist circumference, glycemia, HbA1c, ACTH levels and insulin resistance indexes than DM− (p < 0.05). Moreover, DM+ patients exhibited increased C-peptide (p = 0.004) and glucose area under the curve (AUC) (p = 0.021) during MMTT, with a blunted insulinotropic peptide (GIP) response (p = 0.035). Glucagon levels were similar in both groups, showing a quick rise after meals. No difference in estimated insulin secretion and insulin:glucagon ratio was found. After two months, PAS induced an increase in both fasting glycemia and HbA1c compared to baseline (p < 0.05). However, this glucose trend after meal did not worsen despite the blunted insulin and C-peptide response to MMTT. After PAS treatment, patients exhibited reduced insulin secretion (p = 0.005) and resistance (p = 0.007) indexes. Conversely, glucagon did not change with a consequent impairment of insulin:glucagon ratio (p = 0.009). No significant differences were observed in incretins basal and meal-induced levels. Insulin resistance confirmed its pivotal role in glucocorticoid-induced DM. A blunted GIP response to MMTT in the DM+ group might suggest a potential inhibitory role of hypercortisolism on enteropancreatic axis. As expected, PAS reduced insulin secretion but also induced an improvement in insulin sensitivity as a result of cortisol reduction. No differences in incretin response to MMTT were recorded during PAS therapy. The discrepancy between insulin and glucagon trends while on PAS may be an important pathophysiological mechanism in this iatrogenic DM; hence restoring insulin:glucagon ratio by either enhancing insulin secretion or reducing glucagon tone can be a potential therapeutic target.
Keywords: Cushing’s disease; diabetes mellitus; incretin; mixed meal test tolerance test; pasireotide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

