Characterization of Interactions between CTX-M-15 and Clavulanic Acid, Desfuroylceftiofur, Ceftiofur, Ampicillin, and Nitrocefin
- PMID: 35563620
- PMCID: PMC9100253
- DOI: 10.3390/ijms23095229
Characterization of Interactions between CTX-M-15 and Clavulanic Acid, Desfuroylceftiofur, Ceftiofur, Ampicillin, and Nitrocefin
Abstract
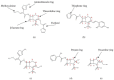
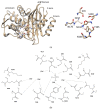
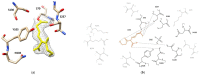
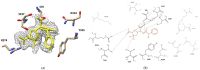
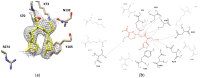
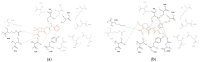
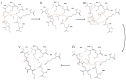
Cefotaximase-Munich (CTX-M) extended-spectrum beta-lactamases (ESBLs) are commonly associated with Gram-negative, hospital-acquired infections worldwide. Several beta-lactamase inhibitors, such as clavulanate, are used to inhibit the activity of these enzymes. To understand the mechanism of CTX-M-15 activity, we have determined the crystal structures of CTX-M-15 in complex with two specific classes of beta-lactam compounds, desfuroylceftiofur (DFC) and ampicillin, and an inhibitor, clavulanic acid. The crystal structures revealed that Ser70 and five other residues (Lys73, Tyr105, Glu166, Ser130, and Ser237) participate in catalysis and binding of those compounds. Based on analysis of steady-state kinetics, thermodynamic data, and molecular docking to both wild-type and S70A mutant structures, we determined that CTX-M-15 has a similar affinity for all beta-lactam compounds (ceftiofur, nitrocefin, DFC, and ampicillin), but with lower affinity for clavulanic acid. A catalytic mechanism for tested β-lactams and two-step inhibition mechanism of clavulanic acid were proposed. CTX-M-15 showed a higher activity toward DFC and nitrocefin, but significantly lower activity toward ampicillin and ceftiofur. The interaction between CTX-M-15 and both ampicillin and ceftiofur displayed a higher entropic but lower enthalpic effect, compared with DFC and nitrocefin. DFC, a metabolite of ceftiofur, displayed lower entropy and higher enthalpy than ceftiofur. This finding suggests that compounds containing amine moiety (e.g., ampicillin) and the furfural moiety (e.g., ceftiofur) could hinder the hydrolytic activity of CTX-M-15.
Keywords: CTX-M-15; antibiotic; antimicrobial resistance; crystal structure; inhibition mechanism; inhibitor; β-lactam compounds; β-lactamase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- CDC . Antibiotic Resistance Threats in the United States. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA, USA: 2019.
-
- Mulvey M.R., Bryce E., Boyd D., Ofner-Agostini M., Christianson S., Simor A.E., Paton S. Ambler Class A Extended-Spectrum Beta-Lactamase-Producing Escherichia coli and Klebsiella spp. in Canadian Hospitals. Antimicrobe Agents Chemother. 2004;48:1204–1214. doi: 10.1128/AAC.48.4.1204-1214.2004. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

