Effects of Long-Term Temozolomide Treatment on Glioblastoma and Astrocytoma WHO Grade 4 Stem-like Cells
- PMID: 35563629
- PMCID: PMC9100657
- DOI: 10.3390/ijms23095238
Effects of Long-Term Temozolomide Treatment on Glioblastoma and Astrocytoma WHO Grade 4 Stem-like Cells
Abstract
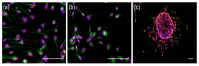
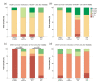
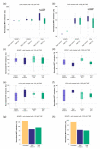
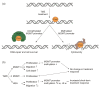
Glioblastoma leads to a fatal course within two years in more than two thirds of patients. An essential cornerstone of therapy is chemotherapy with temozolomide (TMZ). The effect of TMZ is counteracted by the cellular repair enzyme O6-methylguanine-DNA methyltransferase (MGMT). The MGMT promoter methylation, the main regulator of MGMT expression, can change from primary tumor to recurrence, and TMZ may play a significant role in this process. To identify the potential mechanisms involved, three primary stem-like cell lines (one astrocytoma with the mutation of the isocitrate dehydrogenase (IDH), CNS WHO grade 4 (HGA)), and two glioblastoma (IDH-wildtype, CNS WHO grade 4) were treated with TMZ. The MGMT promoter methylation, migration, proliferation, and TMZ-response of the tumor cells were examined at different time points. The strong effects of TMZ treatment on the MGMT methylated cells were observed. Furthermore, TMZ led to a loss of the MGMT promoter hypermethylation and induced migratory rather than proliferative behavior. Cells with the unmethylated MGMT promoter showed more aggressive behavior after treatment, while HGA cells reacted heterogenously. Our study provides further evidence to consider the potential adverse effects of TMZ chemotherapy and a rationale for investigating potential relationships between TMZ treatment and change in the MGMT promoter methylation during relapse.
Keywords: IDH; MGMT; astrocytoma; cancer stem cells; glioblastoma; temozolomide; therapy.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
MeSH terms
Substances
Grants and funding
- none/German National Academic Foundation
- none/Graduate School of Life Sciences Würzburg
- none/Junior clinician scientist fellowship University of Essen
- A21922/Cancer research UK Centre Accelerator Award
- none/UK Department of Health's NIHR Biomedical Research Centre 's funding scheme to University College hospitals NHS foundation trust
LinkOut - more resources
Full Text Sources
Medical
Research Materials

