Recent Progress on the Localization of PLK1 to the Kinetochore and Its Role in Mitosis
- PMID: 35563642
- PMCID: PMC9102930
- DOI: 10.3390/ijms23095252
Recent Progress on the Localization of PLK1 to the Kinetochore and Its Role in Mitosis
Abstract
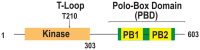
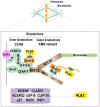
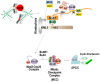
The accurate distribution of the replicated genome during cell division is essential for cell survival and healthy organismal development. Errors in this process have catastrophic consequences, such as birth defects and aneuploidy, a hallmark of cancer cells. PLK1 is one of the master kinases in mitosis and has multiple functions, including mitotic entry, chromosome segregation, spindle assembly checkpoint, and cytokinesis. To dissect the role of PLK1 in mitosis, it is important to understand how PLK1 localizes in the specific region in cells. PLK1 localizes at the kinetochore and is essential in spindle assembly checkpoint and chromosome segregation. However, how PLK1 localizes at the kinetochore remains elusive. Here, we review the recent literature on the kinetochore recruitment mechanisms of PLK1 and its roles in spindle assembly checkpoint and attachment between kinetochores and spindle microtubules. Together, this review provides an overview of how the local distribution of PLK1 could regulate major pathways in mitosis.
Keywords: PLK1 kinase; PP1; PP2A; cell cycle; chromosome segregation; kinetochore; spindle assembly checkpoint.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

