Fluorescence Lifetime Phasor Analysis of the Decamer-Dimer Equilibrium of Human Peroxiredoxin 1
- PMID: 35563654
- PMCID: PMC9100220
- DOI: 10.3390/ijms23095260
Fluorescence Lifetime Phasor Analysis of the Decamer-Dimer Equilibrium of Human Peroxiredoxin 1
Abstract
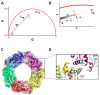
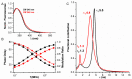
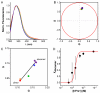
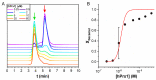
Protein self-assembly is a common feature in biology and is often required for a myriad of fundamental processes, such as enzyme activity, signal transduction, and transport of solutes across membranes, among others. There are several techniques to find and assess homo-oligomer formation in proteins. Naturally, all these methods have their limitations, meaning that at least two or more different approaches are needed to characterize a case study. Herein, we present a new method to study protein associations using intrinsic fluorescence lifetime with phasors. In this case, the method is applied to determine the equilibrium dissociation constant (KD) of human peroxiredoxin 1 (hPrx1), an efficient cysteine-dependent peroxidase, that has a quaternary structure comprised of five head-to-tail homodimers non-covalently arranged in a decamer. The hPrx1 oligomeric state not only affects its activity but also its association with other proteins. The excited state lifetime of hPrx1 has distinct values at high and low concentrations, suggesting the presence of two different species. Phasor analysis of hPrx1 emission lifetime allowed for the identification and quantification of hPrx1 decamers, dimers, and their mixture at diverse protein concentrations. Using phasor algebra, we calculated the fraction of hPrx1 decamers at different concentrations and obtained KD (1.1 × 10-24 M4) and C0.5 (1.36 μM) values for the decamer-dimer equilibrium. The results were validated and compared with size exclusion chromatography. In addition, spectral phasors provided similar results despite the small differences in emission spectra as a function of hPrx1 concentration. The phasor approach was shown to be a highly sensitive and quantitative method to assess protein oligomerization and an attractive addition to the biophysicist's toolkit.
Keywords: dissociation constant; lifetime phasors; peroxiredoxin 1; protein oligomerization; spectral phasors; tryptophan fluorescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

