Hedgehog Signaling Pathway Orchestrates Human Lung Branching Morphogenesis
- PMID: 35563656
- PMCID: PMC9100880
- DOI: 10.3390/ijms23095265
Hedgehog Signaling Pathway Orchestrates Human Lung Branching Morphogenesis
Abstract
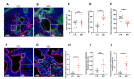
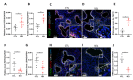
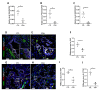
The Hedgehog (HH) signaling pathway plays an essential role in mouse lung development. We hypothesize that the HH pathway is necessary for branching during human lung development and is impaired in pulmonary hypoplasia. Single-cell, bulk RNA-sequencing data, and human fetal lung tissues were analyzed to determine the spatiotemporal localization of HH pathway actors. Distal human lung segments were cultured in an air-liquid interface and treated with an SHH inhibitor (5E1) to determine the effect of HH inhibition on human lung branching, epithelial-mesenchymal markers, and associated signaling pathways in vitro. Our results showed an early and regulated expression of HH pathway components during human lung development. Inhibiting HH signaling caused a reduction in branching during development and dysregulated epithelial (SOX2, SOX9) and mesenchymal (ACTA2) progenitor markers. FGF and Wnt pathways were also disrupted upon HH inhibition. Finally, we demonstrated that HH signaling elements were downregulated in lung tissues of patients with a congenital diaphragmatic hernia (CDH). In this study, we show for the first time that HH signaling inhibition alters important genes and proteins required for proper branching of the human developing lung. Understanding the role of the HH pathway on human lung development could lead to the identification of novel therapeutic targets for childhood pulmonary diseases.
Keywords: Hedgehog pathway; branching; development; human lung.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Mora M.J., Extramiana J., Paniagua P., González P., Mañas A., Pérez M.J., Navarro J., Arrizabalaga M. Orchiectomy and buserelin in combination with flutamide: Comparative results in metastatic prostatic carcinoma. Actas Urol. Esp. 1991;15:548–552. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

