Molecular Research on Oral Diseases and Related Biomaterials: A Journey from Oral Cell Models to Advanced Regenerative Perspectives
- PMID: 35563679
- PMCID: PMC9105421
- DOI: 10.3390/ijms23095288
Molecular Research on Oral Diseases and Related Biomaterials: A Journey from Oral Cell Models to Advanced Regenerative Perspectives
Abstract
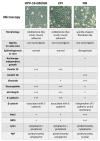
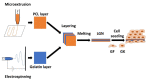
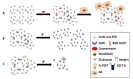
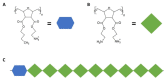
Oral diseases such as gingivitis, periodontitis, and oral cancer affect millions of people worldwide. Much research has been conducted to understand the pathogenetic mechanisms of these diseases and translate this knowledge into therapeutics. This review aims to take the reader on a journey from the initial molecular discoveries to complex regenerative issues in oral medicine. For this, a semi-systematic literature search was carried out in Medline and Web of Science databases to retrieve the primary literature describing oral cell models and biomaterial applications in oral regenerative medicine. First, an in vitro cell model of gingival keratinocytes is discussed, which illustrates patho- and physiologic principles in the context of oral epithelial homeostasis and carcinogenesis and represents a cellular tool to understand biomaterial-based approaches for periodontal tissue regeneration. Consequently, a layered gradient nonwoven (LGN) is described, which demonstrates that the key features of biomaterials serve as candidates for oral tissue regeneration. LGN supports proper tissue formation and obeys the important principles for molecular mechanotransduction. Furthermore, current biomaterial-based tissue regeneration trends, including polymer modifications, cell-based treatments, antimicrobial peptides and optogenetics, are introduced to represent the full spectrum of current approaches to oral disease mitigation and prevention. Altogether, this review is a foray through established and new concepts in oral regenerative medicine and illustrates the process of knowledge translation from basic molecular and cell biological research to future clinical applications.
Keywords: carcinogenesis; cell transformation; mechanotransduction; mesenchymal stem cells; non-woven; tissue homeostasis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Jepsen S., Caton J.G., Albandar J.M., Bissada N.F., Bouchard P., Cortellini P., Demirel K., de Sanctis M., Ercoli C., Fan J. Periodontal manifestations of systemic diseases and developmental and acquired conditions: Consensus report of workgroup 3 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018;45:S219–S229. doi: 10.1111/jcpe.12951. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

