Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate
- PMID: 35563681
- PMCID: PMC9101098
- DOI: 10.3390/cells11091375
Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate
Abstract
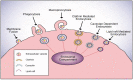
Extracellular vesicles (EVs) are heterogamous lipid bilayer-enclosed membranous structures secreted by cells. They are comprised of apoptotic bodies, microvesicles, and exosomes, and carry a range of nucleic acids and proteins that are necessary for cell-to-cell communication via interaction on the cells surface. They initiate intracellular signaling pathways or the transference of cargo molecules, which elicit pleiotropic responses in recipient cells in physiological processes, as well as pathological processes, such as cancer. It is therefore important to understand the molecular means by which EVs are taken up into cells. Accordingly, this review summarizes the underlying mechanisms involved in EV targeting and uptake. The primary method of entry by EVs appears to be endocytosis, where clathrin-mediated, caveolae-dependent, macropinocytotic, phagocytotic, and lipid raft-mediated uptake have been variously described as being prevalent. EV uptake mechanisms may depend on proteins and lipids found on the surfaces of both vesicles and target cells. As EVs have been shown to contribute to cancer growth and progression, further exploration and targeting of the gateways utilized by EVs to internalize into tumor cells may assist in the prevention or deceleration of cancer pathogenesis.
Keywords: endocytosis; exosomes; extracellular vesicles (EVs); lipids; proteins.
Conflict of interest statement
The authors declare that there are no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

