miR-324-5p and miR-30c-2-3p Alter Renal Mineralocorticoid Receptor Signaling under Hypertonicity
- PMID: 35563683
- PMCID: PMC9104010
- DOI: 10.3390/cells11091377
miR-324-5p and miR-30c-2-3p Alter Renal Mineralocorticoid Receptor Signaling under Hypertonicity
Abstract
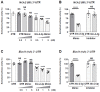
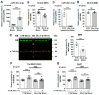
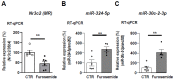
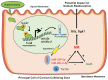
The Mineralocorticoid Receptor (MR) mediates the sodium-retaining action of aldosterone in the distal nephron, but mechanisms regulating MR expression are still poorly understood. We previously showed that RNA Binding Proteins (RBPs) regulate MR expression at the post-transcriptional level in response to variations of extracellular tonicity. Herein, we highlight a novel regulatory mechanism involving the recruitment of microRNAs (miRNAs) under hypertonicity. RT-qPCR validated miRNAs candidates identified by high throughput screening approaches and transfection of a luciferase reporter construct together with miRNAs Mimics or Inhibitors demonstrated their functional interaction with target transcripts. Overexpression strategies using Mimics or lentivirus revealed the impact on MR expression and signaling in renal KC3AC1 cells. miR-324-5p and miR-30c-2-3p expression are increased under hypertonicity in KC3AC1 cells. These miRNAs directly affect Nr3c2 (MR) transcript stability, act with Tis11b to destabilize MR transcript but also repress Elavl1 (HuR) transcript, which enhances MR expression and signaling. Overexpression of miR-324-5p and miR-30c-2-3p alter MR expression and signaling in KC3AC1 cells with blunted responses in terms of aldosterone-regulated genes expression. We also confirm that their expression is increased by hypertonicity in vivo in the kidneys of mice treated with furosemide. These findings may have major implications for the pathogenesis of renal dysfunctions, sodium retention, and mineralocorticoid resistance.
Keywords: aldosterone; hypertonicity; microRNAs; mineralocorticoid receptor; post-transcriptional regulation; sodium reabsorption.
Conflict of interest statement
The authors declare they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

