The Placental NLRP3 Inflammasome and Its Downstream Targets, Caspase-1 and Interleukin-6, Are Increased in Human Fetal Growth Restriction: Implications for Aberrant Inflammation-Induced Trophoblast Dysfunction
- PMID: 35563719
- PMCID: PMC9102093
- DOI: 10.3390/cells11091413
The Placental NLRP3 Inflammasome and Its Downstream Targets, Caspase-1 and Interleukin-6, Are Increased in Human Fetal Growth Restriction: Implications for Aberrant Inflammation-Induced Trophoblast Dysfunction
Abstract
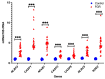
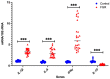
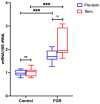
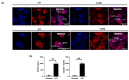
Fetal growth restriction (FGR) is commonly associated with placental insufficiency and inflammation. Nonetheless, the role played by inflammasomes in the pathogenesis of FGR is poorly understood. We hypothesised that placental inflammasomes are differentially expressed and contribute to the aberrant trophoblast function. Inflammasome gene expression profiles were characterised by real-time PCR on human placental tissues collected from third trimester FGR and gestation-matched control pregnancies (n = 25/group). The functional significance of a candidate inflammasome was then investigated using lipopolysaccharide (LPS)-induced models of inflammation in human trophoblast organoids, BeWo cells in vitro, and a murine model of FGR in vivo. Placental mRNA expression of NLRP3, caspases 1, 3, and 8, and interleukin 6 increased (>2-fold), while that of the anti-inflammatory cytokine, IL-10, decreased (<2-fold) in FGR compared with control pregnancies. LPS treatment increased NLRP3 and caspase-1 expression (>2-fold) in trophoblast organoids and BeWo cell cultures in vitro, and in the spongiotrophoblast and labyrinth in the murine model of FGR. However, the LPS-induced rise in NLRP3 was attenuated by its siRNA-induced down-regulation in BeWo cell cultures, which correlated with reduced activity of the apoptotic markers, caspase-3 and 8, compared to the control siRNA-treated cells. Our findings support the role of the NLRP3 inflammasome in the inflammation-induced aberrant trophoblast function, which may contribute to FGR.
Keywords: NLRP3; apoptosis; caspase-1; cytokines; fetal growth restriction; inflammasomes; placental function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Understanding a Potential Role for the NLRP3 Inflammasome in Placenta-Mediated Pregnancy Complications.Am J Reprod Immunol. 2025 Apr;93(4):e70077. doi: 10.1111/aji.70077. Am J Reprod Immunol. 2025. PMID: 40260875 Free PMC article. Review.
-
Formyl peptide receptor-2 is decreased in foetal growth restriction and contributes to placental dysfunction.Mol Hum Reprod. 2018 Feb 1;24(2):94-109. doi: 10.1093/molehr/gax067. Mol Hum Reprod. 2018. PMID: 29272530
-
β-hydroxybutyrate suppresses NLRP3 inflammasome-mediated placental inflammation and lipopolysaccharide-induced fetal absorption.J Reprod Immunol. 2021 Nov;148:103433. doi: 10.1016/j.jri.2021.103433. Epub 2021 Oct 5. J Reprod Immunol. 2021. PMID: 34628106
-
Cholesterol Crystals and NLRP3 Mediated Inflammation in the Uterine Wall Decidua in Normal and Preeclamptic Pregnancies.Front Immunol. 2020 Oct 8;11:564712. doi: 10.3389/fimmu.2020.564712. eCollection 2020. Front Immunol. 2020. PMID: 33117348 Free PMC article.
-
MicroRNAs as important regulators of the NLRP3 inflammasome.Prog Biophys Mol Biol. 2020 Jan;150:50-61. doi: 10.1016/j.pbiomolbio.2019.05.004. Epub 2019 May 15. Prog Biophys Mol Biol. 2020. PMID: 31100298 Review.
Cited by
-
Understanding a Potential Role for the NLRP3 Inflammasome in Placenta-Mediated Pregnancy Complications.Am J Reprod Immunol. 2025 Apr;93(4):e70077. doi: 10.1111/aji.70077. Am J Reprod Immunol. 2025. PMID: 40260875 Free PMC article. Review.
-
Intrauterine Growth Restriction: Need to Improve Diagnostic Accuracy and Evidence for a Key Role of Oxidative Stress in Neonatal and Long-Term Sequelae.Cells. 2024 Mar 13;13(6):501. doi: 10.3390/cells13060501. Cells. 2024. PMID: 38534344 Free PMC article. Review.
-
Melatonin: the placental antioxidant and anti-inflammatory.Front Immunol. 2024 Feb 1;15:1339304. doi: 10.3389/fimmu.2024.1339304. eCollection 2024. Front Immunol. 2024. PMID: 38361952 Free PMC article. Review.
-
Histopathological Clues of Enhanced Inflammation in the Placental Tissue of Women with Chronic Venous Disease in Lower Limbs during Pregnancy.J Pers Med. 2024 Jan 12;14(1):87. doi: 10.3390/jpm14010087. J Pers Med. 2024. PMID: 38248788 Free PMC article.
-
Placentas from Women with Late-Onset Preeclampsia Exhibit Increased Expression of the NLRP3 Inflammasome Machinery.Biomolecules. 2023 Nov 13;13(11):1644. doi: 10.3390/biom13111644. Biomolecules. 2023. PMID: 38002326 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

