Reduced Sarcolemmal Membrane Repair Exacerbates Striated Muscle Pathology in a Mouse Model of Duchenne Muscular Dystrophy
- PMID: 35563723
- PMCID: PMC9100510
- DOI: 10.3390/cells11091417
Reduced Sarcolemmal Membrane Repair Exacerbates Striated Muscle Pathology in a Mouse Model of Duchenne Muscular Dystrophy
Abstract
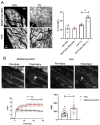
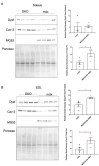
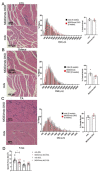
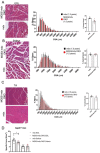
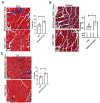
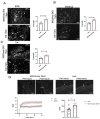
Duchenne muscular dystrophy (DMD) is a common X-linked degenerative muscle disorder that involves mutations in the DMD gene that frequently reduce the expression of the dystrophin protein, compromising the structural integrity of the sarcolemmal membrane and leaving it vulnerable to injury during cycles of muscle contraction and relaxation. This results in an increased frequency of sarcolemma disruptions that can compromise the barrier function of the membrane and lead to death of the myocyte. Sarcolemmal membrane repair processes can potentially compensate for increased membrane disruptions in DMD myocytes. Previous studies demonstrated that TRIM72, a muscle-enriched tripartite motif (TRIM) family protein also known as mitsugumin 53 (MG53), is a component of the cell membrane repair machinery in striated muscle. To test the importance of membrane repair in striated muscle in compensating for the membrane fragility in DMD, we crossed TRIM72/MG53 knockout mice into the mdx mouse model of DMD. These double knockout (DKO) mice showed compromised sarcolemmal membrane integrity compared to mdx mice, as measured by immunoglobulin G staining and ex vivo muscle laser microscopy wounding assays. We also found a significant decrease in muscle ex vivo contractile function as compared to mdx mice at both 6 weeks and 1.5 years of age. As the DKO mice aged, they developed more extensive fibrosis in skeletal muscles compared to mdx. Our findings indicate that TRIM72/MG53-mediated membrane repair can partially compensate for the sarcolemmal fragility associated with DMD and that the loss of membrane repair results in increased pathology in the DKO mice.
Keywords: dystrophy; fibrosis; membrane repair; muscle; sarcolemma.
Conflict of interest statement
N.W. was a founder of TRIM-edicine, Inc., which develops TRIM72/MG53 recombinant protein as a therapeutic approach. All other authors declare that they have no competing interests.
Figures
Similar articles
-
Alterations in Notch signalling in skeletal muscles from mdx and dko dystrophic mice and patients with Duchenne muscular dystrophy.Exp Physiol. 2014 Apr;99(4):675-87. doi: 10.1113/expphysiol.2013.077255. Epub 2014 Jan 17. Exp Physiol. 2014. PMID: 24443351
-
Lipin1 plays complementary roles in myofibre stability and regeneration in dystrophic muscles.J Physiol. 2023 Mar;601(5):961-978. doi: 10.1113/JP284085. Epub 2023 Feb 10. J Physiol. 2023. PMID: 36715084 Free PMC article.
-
Proteomic analysis of the sarcolemma-enriched fraction from dystrophic mdx-4cv skeletal muscle.J Proteomics. 2019 Jan 16;191:212-227. doi: 10.1016/j.jprot.2018.01.015. Epub 2018 Feb 2. J Proteomics. 2019. PMID: 29408692
-
Ion Channels of the Sarcolemma and Intracellular Organelles in Duchenne Muscular Dystrophy: A Role in the Dysregulation of Ion Homeostasis and a Possible Target for Therapy.Int J Mol Sci. 2023 Jan 23;24(3):2229. doi: 10.3390/ijms24032229. Int J Mol Sci. 2023. PMID: 36768550 Free PMC article. Review.
-
The role of branched fibres in the pathogenesis of Duchenne muscular dystrophy.Exp Physiol. 2011 Jun;96(6):564-71. doi: 10.1113/expphysiol.2010.056713. Epub 2011 Mar 18. Exp Physiol. 2011. PMID: 21421700 Review.
Cited by
-
Voluntary wheel running improves molecular and functional deficits in a murine model of facioscapulohumeral muscular dystrophy.iScience. 2023 Dec 2;27(1):108632. doi: 10.1016/j.isci.2023.108632. eCollection 2024 Jan 19. iScience. 2023. PMID: 38188524 Free PMC article.
-
Whole-exome sequencing identifies TRIM72 as a candidate gene for autosomal recessive limb-girdle muscular dystrophy.Hum Genomics. 2025 Aug 13;19(1):91. doi: 10.1186/s40246-025-00809-7. Hum Genomics. 2025. PMID: 40804694 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

