The Role of Extracellular Vesicles in Metabolic Reprogramming of the Tumor Microenvironment
- PMID: 35563739
- PMCID: PMC9104192
- DOI: 10.3390/cells11091433
The Role of Extracellular Vesicles in Metabolic Reprogramming of the Tumor Microenvironment
Abstract
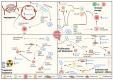
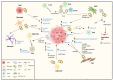
The tumor microenvironment (TME) includes a network of cancerous and non-cancerous cells, together with associated blood vessels, the extracellular matrix, and signaling molecules. The TME contributes to cancer progression during various phases of tumorigenesis, and interactions that take place within the TME have become targets of focus in cancer therapy development. Extracellular vesicles (EVs) are known to be conveyors of genetic material, proteins, and lipids within the TME. One of the hallmarks of cancer is its ability to reprogram metabolism to sustain cell growth and proliferation in a stringent environment. In this review, we provide an overview of TME EV involvement in the metabolic reprogramming of cancer and stromal cells, which favors cancer progression by enhancing angiogenesis, proliferation, metastasis, treatment resistance, and immunoevasion. Targeting the communication mechanisms and systems utilized by TME-EVs is opening a new frontier in cancer therapy.
Keywords: cancer metabolism; exosomes; extracellular vesicles (EVs); glycolysis; tumor microenvironment (TME).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Landscape of extracellular vesicles in the tumour microenvironment: Interactions with stromal cells and with non-cell components, and impacts on metabolic reprogramming, horizontal transfer of neoplastic traits, and the emergence of therapeutic resistance.Semin Cancer Biol. 2021 Sep;74:24-44. doi: 10.1016/j.semcancer.2021.01.007. Epub 2021 Feb 2. Semin Cancer Biol. 2021. PMID: 33545339 Review.
-
Extracellular Vesicles in the Tumour Microenvironment: Eclectic Supervisors.Int J Mol Sci. 2020 Sep 15;21(18):6768. doi: 10.3390/ijms21186768. Int J Mol Sci. 2020. PMID: 32942702 Free PMC article. Review.
-
Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression.Signal Transduct Target Ther. 2020 Oct 19;5(1):242. doi: 10.1038/s41392-020-00359-5. Signal Transduct Target Ther. 2020. PMID: 33077737 Free PMC article. Review.
-
The role of extracellular vesicles from different origin in the microenvironment of head and neck cancers.Mol Cancer. 2019 Apr 6;18(1):83. doi: 10.1186/s12943-019-0985-3. Mol Cancer. 2019. PMID: 30954079 Free PMC article. Review.
-
Emerging Role of Extracellular Vesicles and Cellular Communication in Metastasis.Cells. 2021 Dec 6;10(12):3429. doi: 10.3390/cells10123429. Cells. 2021. PMID: 34943937 Free PMC article. Review.
Cited by
-
Editorial: Extracellular vesicles as modulators of cancer cell adaptive responses linked to therapy resistance.Front Oncol. 2022 Nov 29;12:1101103. doi: 10.3389/fonc.2022.1101103. eCollection 2022. Front Oncol. 2022. PMID: 36523964 Free PMC article. No abstract available.
-
Cancer EV stimulate endothelial glycolysis to fuel protein synthesis via mTOR and AMPKα activation.J Extracell Vesicles. 2024 Jul;13(7):e12449. doi: 10.1002/jev2.12449. J Extracell Vesicles. 2024. PMID: 39001708 Free PMC article.
-
Extracellular Vesicles in Cancer Drug Resistance: Implications on Melanoma Therapy.Cancers (Basel). 2023 Feb 8;15(4):1074. doi: 10.3390/cancers15041074. Cancers (Basel). 2023. PMID: 36831417 Free PMC article. Review.
-
Extracellular vesicles as tools and targets in therapy for diseases.Signal Transduct Target Ther. 2024 Feb 5;9(1):27. doi: 10.1038/s41392-024-01735-1. Signal Transduct Target Ther. 2024. PMID: 38311623 Free PMC article. Review.
-
The signature of extracellular vesicles in hypoxic breast cancer and their therapeutic engineering.Cell Commun Signal. 2024 Oct 21;22(1):512. doi: 10.1186/s12964-024-01870-w. Cell Commun Signal. 2024. PMID: 39434182 Free PMC article. Review.
References
-
- Stincone A., Prigione A., Cramer T., Wamelink M.M.C., Campbell K., Cheung E., Olin-Sandoval V., Grüning N.-M., Krüger A., Tauqeer Alam M., et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015;90:927–963. doi: 10.1111/brv.12140. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

