Schwann Cells Promote Myogenic Differentiation of Myoblasts and Adipogenic Mesenchymal Stromal Cells on Poly-ɛ-Caprolactone-Collagen I-Nanofibers
- PMID: 35563742
- PMCID: PMC9100029
- DOI: 10.3390/cells11091436
Schwann Cells Promote Myogenic Differentiation of Myoblasts and Adipogenic Mesenchymal Stromal Cells on Poly-ɛ-Caprolactone-Collagen I-Nanofibers
Abstract
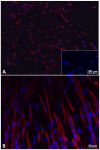
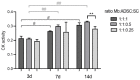
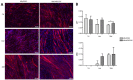
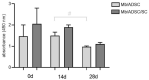
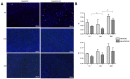
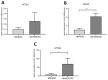
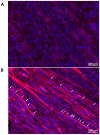
For the purpose of skeletal muscle tissue engineering, different cell types have been investigated regarding their myogenic differentiation potential, including co-cultured myoblasts and adipogenic mesenchymal stromal cells (Mb/ADSC). As neural cells enhance synaptic junction formation, the aim of this study was to co-culture Schwann cells (SCs) with Mb/ADSC on biocompatible electrospun aligned poly-ε-polycaprolacton (PCL)-collagen I-nanofibers. It was hypothesized that SCs, as part of the peripheral nervous system, promote the myogenic differentiation of Mb/ADSC co-cultures. Mb/ADSC were compared to Mb/ADSC/SC regarding their capacity for myogenic differentiation via immunofluorescent staining and gene expression of myogenic markers. Mb/ADSC/SC showed more myotubes after 28 days of differentiation (p ≤ 0.05). After 28 days of differentiation on electrospun aligned PCL-collagen I-nanofibers, gene expression of myosin heavy chains (MYH2) and myogenin (MYOG) was upregulated in Mb/ADSC/SC compared to Mb/ADSC (p ≤ 0.01 and p ≤ 0.05, respectively). Immunofluorescent staining for MHC showed highly aligned multinucleated cells as possible myotube formation in Mb/ADSC/SC. In conclusion, SCs promote myogenic differentiation of Mb/ADSC. The co-culture of primary Mb/ADSC/SC on PCL-collagen I-nanofibers serves as a physiological model for skeletal muscle tissue engineering, applicable to future clinical applications.
Keywords: ADSC; Schwann cells; mesenchymal stem cells; myoblasts; myogenic differentiation; nanofibers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Myogenic differentiation of human myoblasts and Mesenchymal stromal cells under GDF11 on NPoly-ɛ-caprolactone-collagen I-Polyethylene-nanofibers.BMC Mol Cell Biol. 2023 May 15;24(1):18. doi: 10.1186/s12860-023-00478-1. BMC Mol Cell Biol. 2023. PMID: 37189080 Free PMC article.
-
Myogenic differentiation of primary myoblasts and mesenchymal stromal cells under serum-free conditions on PCL-collagen I-nanoscaffolds.BMC Biotechnol. 2018 Nov 26;18(1):75. doi: 10.1186/s12896-018-0482-6. BMC Biotechnol. 2018. PMID: 30477471 Free PMC article.
-
Schwann Cells Do Not Promote Myogenic Differentiation in the EPI Loop Model.Tissue Eng Part A. 2024 Mar;30(5-6):244-256. doi: 10.1089/ten.TEA.2023.0215. Epub 2024 Jan 11. Tissue Eng Part A. 2024. PMID: 38063005
-
Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering.BMC Cell Biol. 2017 Feb 28;18(1):15. doi: 10.1186/s12860-017-0131-2. BMC Cell Biol. 2017. PMID: 28245809 Free PMC article.
-
Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend.Biomaterials. 2007 Jul;28(19):3012-25. doi: 10.1016/j.biomaterials.2007.03.009. Epub 2007 Mar 19. Biomaterials. 2007. PMID: 17408736
Cited by
-
Special Issue "Plastic and Reconstructive Surgery in Personalized Medicine".J Pers Med. 2023 Mar 22;13(3):569. doi: 10.3390/jpm13030569. J Pers Med. 2023. PMID: 36983750 Free PMC article.
-
Myogenic differentiation of human myoblasts and Mesenchymal stromal cells under GDF11 on NPoly-ɛ-caprolactone-collagen I-Polyethylene-nanofibers.BMC Mol Cell Biol. 2023 May 15;24(1):18. doi: 10.1186/s12860-023-00478-1. BMC Mol Cell Biol. 2023. PMID: 37189080 Free PMC article.
-
A Novel Window into Angiogenesis-Intravital Microscopy in the AV-Loop-Model.Cells. 2023 Jan 9;12(2):261. doi: 10.3390/cells12020261. Cells. 2023. PMID: 36672196 Free PMC article.
-
Selection of optimal human myoblasts based on patient related factors influencing proliferation and differentiation capacity.Sci Rep. 2025 Apr 5;15(1):11714. doi: 10.1038/s41598-025-96108-1. Sci Rep. 2025. PMID: 40188257 Free PMC article.
-
Vascularization of Poly-ε-Caprolactone-Collagen I-Nanofibers with or without Sacrificial Fibers in the Neurotized Arteriovenous Loop Model.Cells. 2022 Nov 25;11(23):3774. doi: 10.3390/cells11233774. Cells. 2022. PMID: 36497034 Free PMC article.
References
-
- Knox A.D.C., Ho A.L., Leung L., Tashakkor A.Y., Lennox P.A., Van Laeken N., Macadam S.A. Comparison of Outcomes following Autologous Breast Reconstruction Using the DIEP and Pedicled TRAM Flaps: A 12-Year Clinical Retrospective Study and Literature Review. Plast. Reconstr. Surg. 2016;138:16–28. doi: 10.1097/PRS.0000000000001747. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

