Obesity Affects the Proliferative Potential of Equine Endometrial Progenitor Cells and Modulates Their Molecular Phenotype Associated with Mitochondrial Metabolism
- PMID: 35563743
- PMCID: PMC9100746
- DOI: 10.3390/cells11091437
Obesity Affects the Proliferative Potential of Equine Endometrial Progenitor Cells and Modulates Their Molecular Phenotype Associated with Mitochondrial Metabolism
Abstract
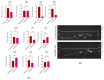
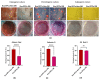
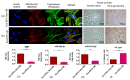
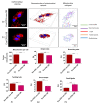
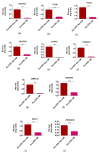
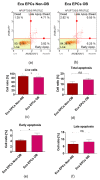
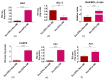
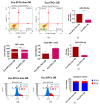
The study aimed to investigate the influence of obesity on cellular features of equine endometrial progenitor cells (Eca EPCs), including viability, proliferation capacity, mitochondrial metabolism, and oxidative homeostasis. Eca EPCs derived from non-obese (non-OB) and obese (OB) mares were characterized by cellular phenotype and multipotency. Obesity-induced changes in the activity of Eca EPCs include the decline of their proliferative activity, clonogenic potential, mitochondrial metabolism, and enhanced oxidative stress. Eca EPCs isolated from obese mares were characterized by an increased occurrence of early apoptosis, loss of mitochondrial dynamics, and senescence-associated phenotype. Attenuated metabolism of Eca EPCs OB was related to increased expression of pro-apoptotic markers (CASP9, BAX, P53, P21), enhanced expression of OPN, PI3K, and AKT, simultaneously with decreased signaling stabilizing cellular homeostasis (including mitofusin, SIRT1, FOXP3). Obesity alters functional features and the self-renewal potential of endometrial progenitor cells. The impaired cytophysiology of progenitor cells from obese endometrium predicts lower regenerative capacity if used as autologous transplants.
Keywords: cellular metabolism; endometrial progenitor cells; obesity; self-renewal potential.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Mitochondrial fission protein 1 up-regulation ameliorates senescence-related endothelial dysfunction of human endothelial progenitor cells.Angiogenesis. 2019 Nov;22(4):569-582. doi: 10.1007/s10456-019-09680-2. Epub 2019 Sep 3. Angiogenesis. 2019. PMID: 31482366
-
N-cadherin identifies human endometrial epithelial progenitor cells by in vitro stem cell assays.Hum Reprod. 2017 Nov 1;32(11):2254-2268. doi: 10.1093/humrep/dex289. Hum Reprod. 2017. PMID: 29040564
-
Endothelial progenitor cells from aged subjects display decreased expression of sirtuin 1, angiogenic functions, and increased senescence.Cell Biol Int. 2018 Sep;42(9):1212-1220. doi: 10.1002/cbin.10999. Epub 2018 Jun 15. Cell Biol Int. 2018. PMID: 29851177
-
Endometrial stem/progenitor cells: the first 10 years.Hum Reprod Update. 2016 Mar-Apr;22(2):137-63. doi: 10.1093/humupd/dmv051. Epub 2015 Nov 9. Hum Reprod Update. 2016. PMID: 26552890 Free PMC article. Review.
-
Endometrial stem/progenitor cells and proliferative disorders of the endometrium.Minerva Ginecol. 2006 Dec;58(6):511-26. Minerva Ginecol. 2006. PMID: 17108881 Review.
Cited by
-
Sex hormone-binding globulin (SHBG) mitigates ER stress and improves viability and insulin sensitivity in adipose-derived mesenchymal stem cells (ASC) of equine metabolic syndrome (EMS)-affected horses.Cell Commun Signal. 2023 Sep 11;21(1):230. doi: 10.1186/s12964-023-01254-6. Cell Commun Signal. 2023. PMID: 37697311 Free PMC article.
-
β-Lactoglobulin affects the oxidative status and viability of equine endometrial progenitor cells via lncRNA-mRNA-miRNA regulatory associations.J Cell Mol Med. 2023 Apr;27(7):927-938. doi: 10.1111/jcmm.17694. Epub 2023 Mar 1. J Cell Mol Med. 2023. PMID: 36860157 Free PMC article.
-
Identification and characterization of stromal-like cells with CD207+/low CD1a+/low phenotype derived from histiocytic lesions - a perspective in vitro model for drug testing.BMC Cancer. 2024 Feb 12;24(1):105. doi: 10.1186/s12885-023-11807-0. BMC Cancer. 2024. PMID: 38342891 Free PMC article.
-
Equine Hoof Progenitor Cells Display Increased Mitochondrial Metabolism and Adaptive Potential to a Highly Pro-Inflammatory Microenvironment.Int J Mol Sci. 2023 Jul 14;24(14):11446. doi: 10.3390/ijms241411446. Int J Mol Sci. 2023. PMID: 37511204 Free PMC article.
-
The Molecular Link between Obesity and the Endometrial Environment: A Starting Point for Female Infertility.Int J Mol Sci. 2024 Jun 22;25(13):6855. doi: 10.3390/ijms25136855. Int J Mol Sci. 2024. PMID: 38999965 Free PMC article. Review.
References
-
- Robles M., Nouveau E., Gautier C., Mendoza L., Dubois C., Dahirel M., Lagofun B., Aubrière M.-C., Lejeune J.-P., Caudron I., et al. Maternal Obesity Increases Insulin Resistance, Low-Grade Inflammation and Osteochondrosis Lesions in Foals and Yearlings until 18 Months of Age. PLoS ONE. 2018;13:e0190309. doi: 10.1371/journal.pone.0190309. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

