HSP90 Modulates T2R Bitter Taste Receptor Nitric Oxide Production and Innate Immune Responses in Human Airway Epithelial Cells and Macrophages
- PMID: 35563784
- PMCID: PMC9101439
- DOI: 10.3390/cells11091478
HSP90 Modulates T2R Bitter Taste Receptor Nitric Oxide Production and Innate Immune Responses in Human Airway Epithelial Cells and Macrophages
Abstract
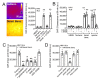
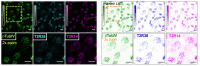
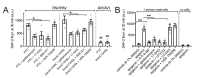
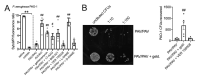
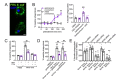
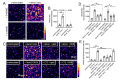
Bitter taste receptors (T2Rs) are G protein-coupled receptors (GPCRs) expressed in various cell types including ciliated airway epithelial cells and macrophages. T2Rs in these two innate immune cell types are activated by bitter products, including those secreted by Pseudomonas aeruginosa, leading to Ca2+-dependent activation of endothelial nitric oxide (NO) synthase (eNOS). NO enhances mucociliary clearance and has direct antibacterial effects in ciliated epithelial cells. NO also increases phagocytosis by macrophages. Using biochemistry and live-cell imaging, we explored the role of heat shock protein 90 (HSP90) in regulating T2R-dependent NO pathways in primary sinonasal epithelial cells, primary monocyte-derived macrophages, and a human bronchiolar cell line (H441). Immunofluorescence showed that H441 cells express eNOS and T2Rs and that the bitter agonist denatonium benzoate activates NO production in a Ca2+- and HSP90-dependent manner in cells grown either as submerged cultures or at the air-liquid interface. In primary sinonasal epithelial cells, we determined that HSP90 inhibition reduces T2R-stimulated NO production and ciliary beating, which likely limits pathogen clearance. In primary monocyte-derived macrophages, we found that HSP-90 is integral to T2R-stimulated NO production and phagocytosis of FITC-labeled Escherichia coli and pHrodo-Staphylococcus aureus. Our study demonstrates that HSP90 serves as an innate immune modulator by regulating NO production downstream of T2R signaling by augmenting eNOS activation without impairing upstream Ca2+ signaling. These findings suggest that HSP90 plays an important role in airway antibacterial innate immunity and may be an important target in airway diseases such as chronic rhinosinusitis, asthma, or cystic fibrosis.
Keywords: airway epithelium; bitter taste receptors; calcium; cilia; heat shock proteins; innate immunity; macrophages; nitric oxide; sinusitis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


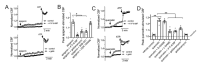


Similar articles
-
Loss of CFTR function is associated with reduced bitter taste receptor-stimulated nitric oxide innate immune responses in nasal epithelial cells and macrophages.Front Immunol. 2023 Jan 18;14:1096242. doi: 10.3389/fimmu.2023.1096242. eCollection 2023. Front Immunol. 2023. PMID: 36742335 Free PMC article.
-
Activation of airway epithelial bitter taste receptors by Pseudomonas aeruginosa quinolones modulates calcium, cyclic-AMP, and nitric oxide signaling.J Biol Chem. 2018 Jun 22;293(25):9824-9840. doi: 10.1074/jbc.RA117.001005. Epub 2018 May 10. J Biol Chem. 2018. PMID: 29748385 Free PMC article.
-
Bitter taste receptors stimulate phagocytosis in human macrophages through calcium, nitric oxide, and cyclic-GMP signaling.Cell Mol Life Sci. 2021 Jan;78(1):271-286. doi: 10.1007/s00018-020-03494-y. Epub 2020 Mar 14. Cell Mol Life Sci. 2021. PMID: 32172302 Free PMC article.
-
Sinonasal solitary chemosensory cells "taste" the upper respiratory environment to regulate innate immunity.Am J Rhinol Allergy. 2014 Sep-Oct;28(5):366-73. doi: 10.2500/ajra.2014.28.4077. Am J Rhinol Allergy. 2014. PMID: 25198020 Review.
-
The role of bitter and sweet taste receptors in upper airway innate immunity: Recent advances and future directions.World J Otorhinolaryngol Head Neck Surg. 2018 Aug 24;4(3):200-208. doi: 10.1016/j.wjorl.2018.07.003. eCollection 2018 Sep. World J Otorhinolaryngol Head Neck Surg. 2018. PMID: 30506052 Free PMC article. Review.
Cited by
-
Genetic variants in taste genes play a role in oral microbial composition and severe early childhood caries.iScience. 2022 Nov 9;25(12):105489. doi: 10.1016/j.isci.2022.105489. eCollection 2022 Dec 22. iScience. 2022. PMID: 36404915 Free PMC article.
-
Interkingdom Detection of Bacterial Quorum-Sensing Molecules by Mammalian Taste Receptors.Microorganisms. 2023 May 16;11(5):1295. doi: 10.3390/microorganisms11051295. Microorganisms. 2023. PMID: 37317269 Free PMC article. Review.
-
Akt activator SC79 stimulates antibacterial nitric oxide generation in human nasal epithelial cells in vitro.Int Forum Allergy Rhinol. 2024 Jul;14(7):1147-1162. doi: 10.1002/alr.23318. Epub 2024 Jan 10. Int Forum Allergy Rhinol. 2024. PMID: 38197521 Free PMC article.
-
Cellular mechanisms and molecular pathways linking bitter taste receptor signalling to cardiac inflammation, oxidative stress, arrhythmia and contractile dysfunction in heart diseases.Inflammopharmacology. 2023 Feb;31(1):89-117. doi: 10.1007/s10787-022-01086-9. Epub 2022 Dec 6. Inflammopharmacology. 2023. PMID: 36471190 Free PMC article. Review.
-
Oxidative Stress and Air Pollution: Its Impact on Chronic Respiratory Diseases.Int J Mol Sci. 2023 Jan 3;24(1):853. doi: 10.3390/ijms24010853. Int J Mol Sci. 2023. PMID: 36614301 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

